Dr William O’Neill is the Medical Director of the Henry Ford Health System and pioneered the use of angioplasty in heart attack treatment. In the field of structural heart disease, he performed the first transcatheter aortic valve replacement procedure in the US in 2005. He has received numerous awards and has authored more than 300 peer-reviewed articles and abstracts.
Dr O’Neill began his presentation by highlighting the need for improved outcomes in cardiogenic shock (CS). Mortality in CS used to be as high as 90 % in the 1960s. Thirty years ago, the first studies reporting the outcomes of angioplasty for CS were published. Results showed a survival rate of 50 %. Survival has not improved since then. A recent cohort study found that long-term outcomes in CS remain poor.1 This represents a clear, unmet need.
While the advent of mechanical circulatory support (MCS) devices offers promise in terms of improving outcomes in CS patients, there is a need for more evidence in support of their use. The catheterbased Ventricular Assist Device Registry™ (cVAD Registry™) is a global observational clinical registry designed to monitor patient safety and real-world outcomes of patients supported with the Impella® (Abiomed) device. Dr O’Neill presented registry data from his patients who were in severe haemodynamic compromise. These data showed that the use of increasing numbers of inotropes prior to MCS implantation is associated with worse survival and may increase the size of an infarct.
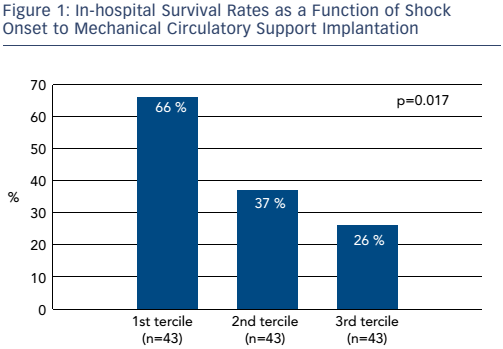
The time between onset of CS and initiation of MCS is also an important determinant of survival. Figure 1 shows in-hospital survival rates as a function of shock onset to MCS initiation in a cohort of 129 patients. In the first tercile (n=43), the time from onset of shock to support was 1.5 hours. In the second tercile (n=43), the time was between 1.5 and 4.0 hours and in the third tercile (n=43) it was >4 hours. A very steep gradient of survival versus time to onset of support was seen. If CS occurred >4 hours before MCS was initiated, survival was only 26 %; while survival was 66 % if CS patients received MCS within 1.5 hours from the onset of CS. In Dr O’Neill’s experience, patients are delayed for too long before being transferred to specialist centres. The resulting prolongation of CS may lead to irreversible end-organ damage.
Dr O’Neill shared his research into clinical outcomes based on whether patients had the Impella device implanted before or after coronary reperfusion in the setting of acute myocardial infarction (AMI) complicated by CS.2 This work showed there was a clear advantage to initiating Impella support prior to percutaneous coronary intervention. The separation of the curves occurs very early after percutaneous coronary intervention, reinforcing the belief that early MCS initiation is a key determinant of clinical outcomes.
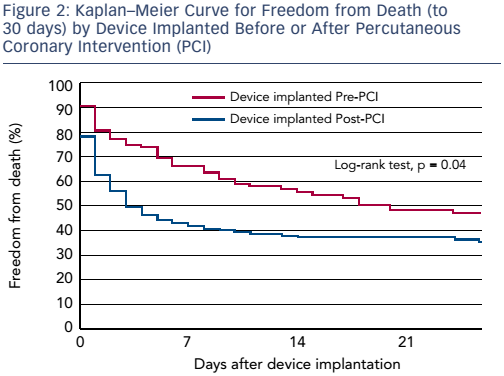
These data highlight the need for a paradigm shift in the management of CS. Dr O’Neill asserted that interventionalists need to shift their thinking from door-to-balloon time to door-to-support time. The initiation of Impella prior to reperfusion may prolong the overall doorto-balloon time, but this delay is probably justified in the settings of AMI-CS as it provides end-organ perfusion and cardiac unloading.
Dr O’Neill was asked which he would consider a priority for a randomised controlled trial. In reply, he pointed out that 55 % of CS patients in the cVAD Registry would be ineligible for a clinical trial because of exclusion criteria such as out-of-hospital cardiac arrest. In a high-risk situation that needs immediate action, there is no time to speak to family members and obtain consent for inclusion in a randomised trial. While the latter may be possible for haemodynamically-stable AMI patients, registries are a better option to assess outcomes in CS. As the cVAD Registry continues to accrue data, Dr O’Neill expects to see an increased proportion of patients receiving MCS prior to coronary reperfusion, with corresponding improvements in survival.








