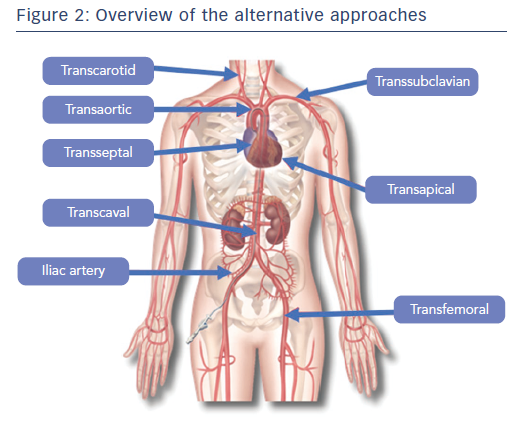
This article aims to provide an updated comparative review, addressing the respective strengths and weaknesses of different alternative transcatheter aortic valve implantation (TAVI) approaches.
The transfemoral (TF) access is favoured by international guidelines on TAVI because of its reported superiority to the transthoracic approach.1–3 In spite of the progress in miniaturisation of new-generation transcatheter heart valves (THV), comorbidities and unfavourable anatomy preclude TF access in 15–20 % of patients according to contemporary registries.4,5 Besides, vascular complications with the TF approach are as high as 6 % in recent randomised Placement of Aortic Transcatheter Valves 2 (PARTNER 2) and Surgical Replacement and Transcatheter Aortic Valve Implantation (SURTAVI) trials, which reflects that some patients could benefit from an alternative approach. Historically, the transapical (TA) approach was the first to be introduced for broad clinical use in TAVI.6 Newer and less invasive pathways have since been proposed and developed, namely transcarotid (TC), transaortic (TAo), trans-subclavian (TS) and transcaval (TCv). However, the relative ‘invasiveness’ of one alternative approach compared with another is subject to debate. Invasiveness relates to the need for a surgical cutdown, general anaesthesia, vascular or heart lesion required for delivery system crossing, and potential impact on the other major systems such as the cerebral, respiratory and renal systems.
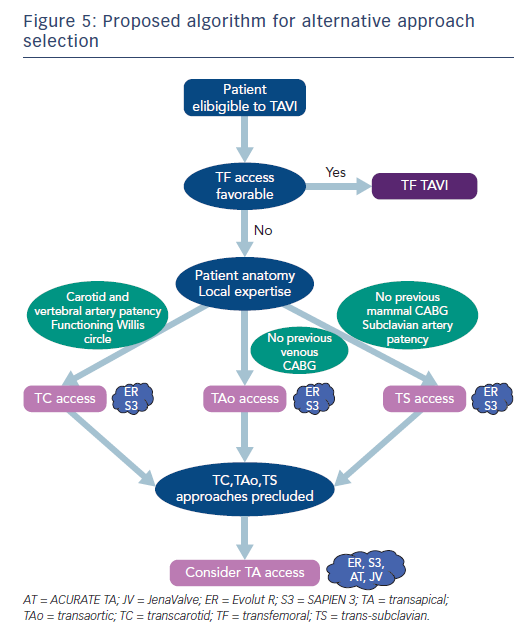
No randomised trial directly compares the different approaches, and these alternative approaches are more or less subdued to a learning curve.7 Also, patient anatomy and comorbidities often determine eligibility for alternative pathways. Indeed, general anaesthesia for TA or TAo access may be contraindicated in patients with chronic respiratory insufficiency and the TS pathway may be precluded by vascular anatomy, such as tortuosity, stenosis or angulation. Patients who have previously undergone a coronary artery bypass may be unsuitable for the TAo pathway if they received venous grafts, or for the TS pathway if they received mammal artery grafts.
Transthoracic Approaches
Transapical TAVI
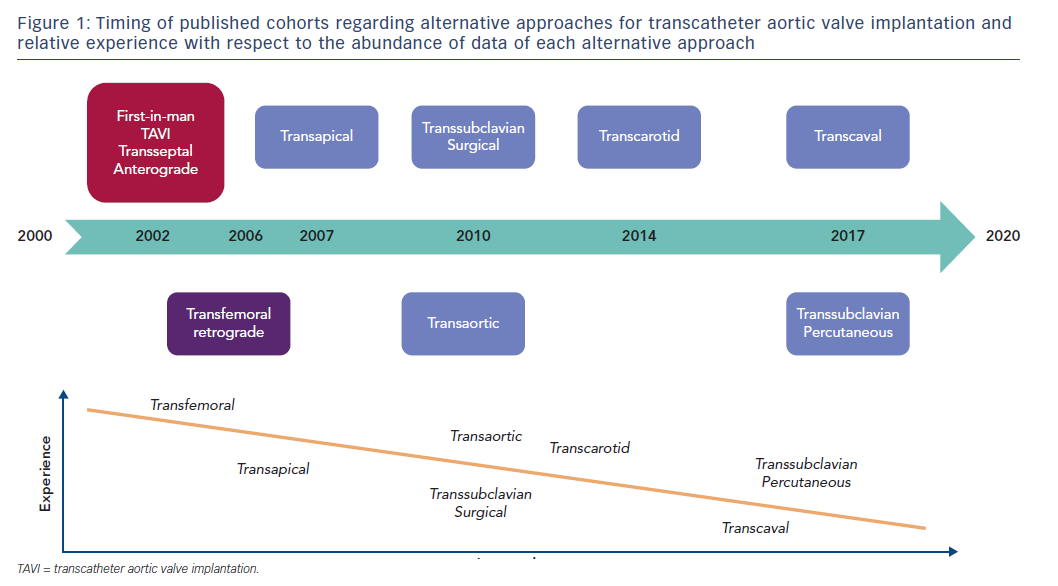
The TA pathway was the first alternative described for when the TF approach is not possible. Therefore, this is the alternative pathway with most abundant data (Figure 1).8 The TA pathway is the only anterograde TAVI approach; it provides easy valve crossing and excellent controllability.9 TA TAVI avoids cardiopulmonary bypass and sternotomy, as it is a beating-heart left transthoracic approach (Figure 2).10 However, TA access requires a surgical cutdown through anterolateral mini-thoracotomy with general anaesthesia and mechanical ventilation. Furthermore, the impact of the crossing by the delivery system through the left ventricle apex and closing after the implantation has been reported to be responsible for apex myocardium stunning and possibly necrosis, which impairs left ventricle function.11
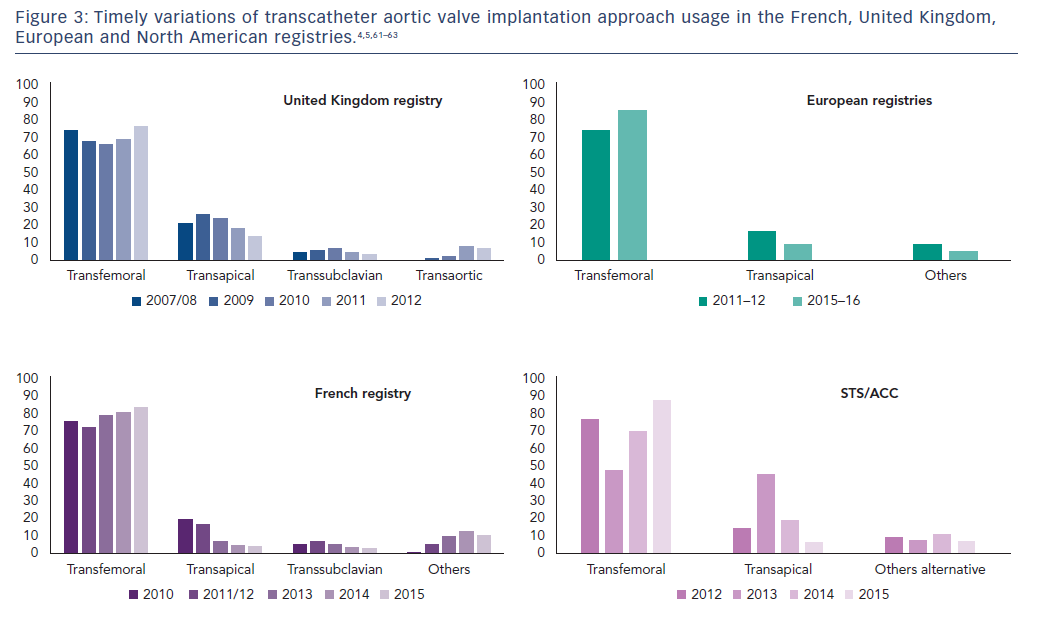
These drawbacks encouraged operators to master other less morbid alternative pathways, and the TA pathway’s use has decreased over time. Indeed, TA access was undertaken in 17.6 % of patients in the early FRench Aortic National CoreValve and Edwards (FRANCE) 2 registry (2010–2012) compared with 4.2 % in the more recent FRANCE TAVI registry (2013–2015). A similar reduction in popularity was noted overseas as reported in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry (Figure 3).4,5
Comparisons between TA and TF TAVI or surgical aortic valve replacement (SAVR) are based on observational prospective studies. TA is inferior to TF TAVI. Indeed, in inoperable and high-risk patients the 30-day mortality with TA TAVI has been reported to be close to 5.0 %12,13 compared with 1.6 % for the TF approach in contemporary registries.13 Furthermore, one-year survival was 72 % after TA versus 81 % after TF TAVI in the SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry.14 TA access was associated with particularly worse outcomes in nonagerians; 30-day mortality was as high as 14.2 % (versus 6.5 % with the TF access, HR 2.56; 95 % CI [1.63–4.01])15–17 in a recent meta-analysis of three studies. Vascular complications are twice as few with the TA approach but the rate of major bleeding was twice as high.13 On the other hand, one recent cohort of intermediate- and low-risk patients, showed similar 30-day mortality (1.5 %), stroke (3.5 %) and pacemaker implantation (10.0 %),13 underscoring the need for further research. These remarks should be tempered by the fact that patients treated with TA TAVI often share more comorbidities than those treated with the TF approach. However, comparisons after propensity-matching seem to support these data.18
TA TAVI yields comparable results to SAVR in inoperable and high-risk patients. One retrospective study with pair matching of 164 high-risk patients (mean logistic EuroScore over 29 %) reported similar one-year survival rates for TA TAVI and SAVR (81 % versus 76 %, respectively; p=0.66). Stroke, pacemaker and indexes of physical and mental quality of life were also similar.19 However, the five-year mortality in high-risk patients favoured SAVR (HR 1.37; 95 % CI [0.98–1.92]).20 In the PARTNER 2 trial that randomised intermediate- and low-risk patients, the outcome of the transthoracic approach group (pooling of patients treated through TA and TAo pathways), was found to be non-inferior to SAVR (HR of composite primary outcome including all-cause death and disabling stroke 1.21; 95 % CI [0.84–1.74]).1 In another cohort, with a long five-year follow-up of low-risk patients, similar survival rates (86 % after SAVR versus 76 % after TA TAVI) were reported, along with similar health related physical or mental quality of life.21 Conversely, the STACCATO trial, which randomised elderly low-risk patients was prematurely stopped after including only 72 patients because of excessive adverse events (death, stroke, acute renal failure, severe paravalvular leakages) in the TA TAVI group.22
A meta-analysis that pooled data from randomised trials with all operative-risk patients (PARTNER 2A, The Nordic aortic valve intervention [NOTION], US CoreValve High Risk and STACCATO or PARTNER 1A) found no difference in two-year mortality risk between TA TAVI and SAVR. Similarly, stroke (RR 1.67; 95 % CI [0.97–2.87]) and acute kidney injury (RR 1.54; 95 % CI [0.77–3.07]) were not different. However, life threatening or disabling bleeding were less frequently observed with TA TAVI (RR 0.53; 95 % CI [0.42–0.67]).1,22–25
Most of these studies included patients treated with the Edwards SAPIEN (Edwards Lifesciences, Irvine, CA, USA) and Medtronic CoreValve (Medtronic, Minneapolis, MN, USA) transcatheter heart valves (THV). However, data are concordant with newer-generation devices. A recent international registry reported results with the TA implanted ACURATE TA™ THV in 500 high-risk patients (Society of Thoracic Surgeons score; STS 9.8 ± 8.3 %). Mortality was high (7 % at 30 days and 20 % at 1 year) albeit comparative to registries involving other THV types and similar patient operative risk, the stroke rate was low (2.2 % at 30 days, 3.7 % at one year). Also, only 10 % of patients required a permanent pacemaker and 2 % of patients had echocardiographic paravalvular leakage ≥2+ (none were 3+ or 4+).26,27 These results are encouraging and the TF implantable version of the ACURATE THV (ACURATE Neo™), might benefit from a broader adoption by avoiding the TA pathway drawbacks.28,29 Data with other new-generation devices are reassuring SAPIEN 3, ACURATE TA, Engager, JenaValve™. The design of this new-generation of THV is interesting and yields low rates of prosthetic regurgitation, but they are not likely to enjoy broad adoption because of the high mortality of the TA approach.13,27,30–33
TA access is being progressively abandoned by TAVI operators because it requires a surgical cutdown, general anaesthesia and intubation. Also, there is growing evidence of inferiority to other pathways and open surgery in the elderly as well as lower-risk patients.
Transaortic Access
TAo (or direct aortic) access requires a partial sternotomy or mini-thoracotomy and no cardiopulmonary bypass as opposed to SAVR. After a 4–5 cm skin incision, a reverse T- or right J-shaped sternotomy down to the second intercostal space or mini-right thoracotomy in the second intercostal space (according to the position of the aorta regarding the sternum on pre-TAVI multi-slice CT) is performed, before puncturing the aorta.34,35 This approach allows excellent controllability and precise THV positioning. SAPIEN and CoreValve THVs have been the most explored devices with the TAo approach.36
TAo approach has often been presented as an alternative to TA when TF access is precluded.34 Observational data with high-risk patients suggest that TAo yields lower short-term as well as long-term mortality than the TA access.36–39 The risk of stroke is comparable to TF, but the invasive surgical access of the TAo approach yields high rates of life-threatening bleeding complications that are comparable to TA.37,39 In a recent observational study, Arai et al. provided a univariate comparison of 467 patients treated with TF to 289 patients treated with TAo and 42 patients treated with TA approaches. They found 30-day mortality to be 9 % for TAo versus 14 % for TA and 5 % for TF and one-year mortality of 20 % after TAo versus 30 % after TA and 13 % after TF. Strokes were fewer with TF and TAo (both 2 %) than TA (5 %). Acute kidney injury rates were lower after TAo compared with TA, but still higher than TF (13 % versus 31 % versus 5 %, respectively).37 Because no multivariable analysis was performed, these results should be considered with care. These results were consistent with those of McCarthy et al. who also found similar healthcare costs for TAo and TA approaches, although both were more costly than TF, which was partly a result of longer hospital stays.40 A more recent multicentre study reported further favourable outcomes after TAo TAVI with 6.1 % and 1.0 % mortality and stroke rates, respectively.41 Recent propensity-matched data in lower-risk patients are concordant.42 These results support the superiority of TAo over TA access, but not against TF. Indeed, major or life-threatening bleeding at 30 days has recently been reported to be as high as 66.7 % after TAo TAVI, twice as much as in a propensity-matched TF group.39 A meta-analysis by Amrane et al. found concordant 10.0 % 30-day mortality rate across 15 studies and 3.7 % strokes across nine studies.36 The patients included in studies of the TAo approach have mostly been of high surgical risk and further validation in lower-risk categories is warranted. TAo is also yet to be compared with non-TA alternative approaches in methodologically robust studies.
Extra-thoracic Approaches
Transcarotid Approach
The TC approach was first described in 2010.43 Experience with carotid access is important for the surgeon to provide optimal results with the TC approach because of specific anatomical challenges such as the presence of the vagus nerve and the respiratory tract. Theoretically, the left carotid access for TAVI should be favoured over the right because it provides superior coaxial alignment with the ascending aorta and optimal positioning for the THV during the device deployment. However, right carotid access has also been successfully used.44,45 Data from the recent FRANCE TAVI registry showed that up to 3.4 % of patients are now treated with the TC approach.5 The TC access has the potential to alleviate some of drawbacks of the other non-femoral approaches, given its minimally-invasive nature.35,46–48 Previous reports demonstrated the feasibility and safety of the TC approach, mostly with the Medtronic CoreValve THV.43,49,50 Our experience with the SAPIEN devices is also reassuring.
The minimally-invasive surgery of the TC pathway has been mastered by numerous heart teams and recently it has been reported to have similar outcomes to TF access in terms of mortality and morbidity.49 Recent studies reported a mortality of 6.3 % at 30 days and 16.7 % at one year. Furthermore, rates of 30-day cerebrovascular events in our registry were similar to existing data with transfemoral TAVI,51 in particular the rate of 30-day stroke was under 2.5 % with TC TAVI.44,49,50 Preference for local anaesthesia with conscious sedation to general anaesthesia is team-dependent. Azmoun et al. observed in-hospital death in two of the 19 patients treated with TC TAVI under local anaesthesia in their cohort.50 One larger registry reported lower numerical rates of 30-day strokes with local anaesthesia and conscious sedation than general anaesthesia (0.0 % versus 2.2 %), albeit without statistical significance.44 Furthermore, the TC approach permits fast ambulation and short hospital stay (median four days) after the intervention.50 Our team has previously demonstrated the effectiveness and safety of the TC TAVI with the Medtronic CoreValve system44,49 and our practice has provided reassuring feedback with the Edwards SAPIEN system as well.
Trans-subclavian
The TS (or transaxillary) approach was one of the first alternative accesses described, and was traditionally considered as mandatory surgical.52,53 The surgical cutdown is performed through an infra-clavicular incision and must respect the brachial plexus.52,54,55 However, a percutaneous approach has recently been proposed.56 Conscious sedation with local anaesthesia or general anaesthesia are possible with the TS pathway.47 Most data on TS access feasibility has been reported with the Medtronic CoreValve and first-generation Edwards SAPIEN THVs, although some recent reports hinted at favourable outcomes with newer generation SAPIEN 3 and Evolut™ R valving systems.57,58 Recent registry data suggest that the use of the TS approach is declining. Indeed, the alternative TS access was performed in 5.8 % of patients in the FRANCE 2 registry (2010-2012) versus 3.0 % in the more recent FRANCE TAVI registry (2013-2015).2
TS was compared with the TF approach in one small propensity-matched cohort, using the CoreValve THV. The authors found a similar two-year survival (approximately 75 %), along with similar rates of procedural success, major vascular and bleeding complications.59 Another more recent observational report of 202 patients compared TA and TS approaches and found no significant association of access site with mortality in the multivariable analysis. Two-year survival was also comparable (84 % after TS and 75 % in TA) although TA yielded higher perioperative mortality; the latter feature could be linked to a higher risk of major and life-threatening bleeding that was less frequent with the TS approach (11.5 %) than the TA approach (40.0 %).47
The percutaneous approach is relatively recent. It has been reported to be feasible and safe in one German cohort of 100 high-risk patients, mostly through left axillary artery and under general anaesthesia. Thirty-day mortality was 6 % and 1-year mortality was 15 %; no stroke was reported.56 The percutaneous TS approach could potentially become a first-choice alternative pathway by avoiding surgical cutdown. However, manual compression is often ineffective for anatomical reasons, exposing patients to potentially fatal bleeding complications. As a result, some additional complex operative steps are required to avoid cataclysmic bleeding, with the insertion of a wire in the ipsilateral brachial artery externalised through the femoral artery or contralateral brachial artery for balloon occlusion or covered stent implantation in case of failure of the percutaneous closure system, such as the Prostar® (Abbott Vascular) or ProGlide (Abbott Vascular).56 Hence, achieving optimal results will require operators to confront a difficult learning curve. Also, further research is required to validate this approach in intermediate- and low-risk patients.
Transcaval
The TCv approach is the most recently described approach for TAVI.60 It aims to avoid the morbidity of the transthoracic (TA and TAo) approaches while providing the favourable operating room ergonomics of the TF access.46 The TCv approach allows large introducer sheath size because of the venous nature of the access without increasing the risk of major bleeding, TCv access use implies the ability to perform a wire crossing from the inferior vena cava into the aorta through the retroperitoneum to allow the passage of the TAVI introducer sheath and delivery system. These steps require precise preoperative planning from the multi-slice CT. Most importantly, the aorta must have few calcifications in the crossing area to avoid tearing of the aortic wall and effective closing of the artificially created cavo-aortic duct after THV implantation. Indeed, the cavo-aortic access requires closing by a cardiac-type occluder device (AMPLATZER™ Duct Occluder or AMPLATZER Ventricular Septal Defect Occluder, St. Jude Medical).46
Experience with the TCv approach was reported in two successive (2013–2014 and 2014–2016) cohorts of 19 and 100 high-risk patients ineligible for the arterial TF as well as TA and TAo pathways, and presenting a suitable cavo-aortic anatomy. The patients were treated with SAPIEN XT and SAPIEN 3 as well as CoreValve and Evolut R THVs, mostly with general anaesthesia although moderate sedation was also allowed.46,60 Retroperitoneal haematoma is the most feared complication specific to the TCv approach. Among the 19 patients of the first cohort, all had persistent aorto-caval flow immediately after the procedure; 16 underwent repeat imaging that showed incomplete closure in one of them.60 In the second cohort, the rate of life-threatening or major bleeding was as high as 18 % and the rate of major vascular complications was as high as 19 %, which underscores the bleeding hazard associated with the TCv approach. Device success was achieved in 98 of 100 patients, eight patients died at 30 days and five presented with a stroke in this cohort of high-risk patients (STS mortality risk score 9.6 ± 6.3 %).46
The TCv approach remains an experimental approach, to be performed by expert centres as an option in patients who are ineligible for TF as well as the other alternative approaches, with abdominal anatomy compatible with a cavo-aortic crossing. The TCv access provides favourable operating room ergonomics and is compatible with conscious sedation. Nevertheless, if sufficient improvements of the technology dedicated to cavo-aortic crossing and closing are achieved, the TCv approach might gain popularity among TAVI operators in the future.
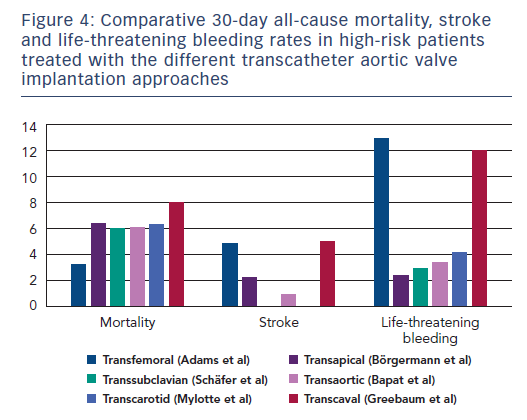
In conclusion, the perfect alternative pathway should be performed percutaneously, under conscious sedation with local anaesthesia, and yield similar results to the arterial TF access regarding the major complications of perioperative mortality, stroke, and major bleeding. None of the existing approaches satisfy all these criteria (Figure 4). As TAVI indications expand to younger and lower-risk patients, perioperative mortality and stroke will become increasingly intolerable, which might encourage TAVI operators to select approaches with the lowest rates of such complications. Hence, when TF access is precluded, TC and TS approaches might benefit from this expansion of indications in the future. Besides these safety concerns, the choice of one alternative access over another is often a matter of anatomical feasibility as assessed by preoperative imaging and local expertise (Figure 5).