Transcatheter aortic valve implantation (TAVI) has emerged as an alternative treatment for symptomatic severe aortic stenosis in patients deemed to be at high operative risk for conventional surgical aortic valve replacement (SAVR). In these patients, TAVI has lower all-cause mortality than SAVR1 and recent data also suggest equipoise between these therapies in intermediate-risk patients.2–5 Similar comparative efficacy trials are under way in patients at low operative risk7–9.
Despite the success of TAVI, serious life-threatening complications can occur. Aortic annular rupture is among the most devastating of these. Although uncommon, the high mortality associated with annular rupture mandates careful procedural planning and execution. This will be especially relevant if TAVI is to be successfully expanded to lower-risk patients.
This article discusses the causes, mechanisms and diagnosis of aortic annular rupture. Techniques to minimise the risk of annular rupture and therapeutic strategies to improve outcomes in patients that experience this complication will be examined.
Anatomy of the Aortic Annulus
The aortic root is the direct continuation of the left ventricular outflow tract (LVOT) and forms a bridge between the left ventricle and the ascending aorta. It functions as the supporting structure for the aortic valve and is comprised of three main components: the sinutubular junction (STJ); the aortic sinuses (consisting of the sinuses of Valsalva, the surgical aortic annulus [ventriculo-aortic junction] and the leaflets of the aortic valve); and the basal ring.
The basal ring, frequently referred to as the “aortic annulus” by those involved in TAVI, is a virtual (rather than anatomic) ring found at the insertion point of the basal attachments of the aortic valve leaflets within the LVOT. Despite the use of the term “annulus”, meaning ring, the annulus is neither circular nor oval.
The aortic valve leaflets arise from their attachment in the muscular LVOT, which forms the haemodynamic ventriculo-arterial junction, and ascend to come together to form the trileaflet valve at the STJ. The sinuses of valsalva lie distal to the semilunar attachments of the leaflets. The left and right facing sinuses give rise to coronary arteries, usually at or below the level of the STJ. The base of the two coronary aortic sinuses have a crescent of myocardium incorporated, though the greater part of the walls of the sinuses are composed of aortic tissue. The STJ represents the zenith of the aortic root which continues as the ascending aorta.
The area of the aortic root and LVOT adjacent to the basal attachment of the valve leaflets is particularly relevant to a discussion on aortic annular rupture. Three triangular fibromuscular extensions, called the interleaflet triangles, are interposed between the leaflets and extend towards the left ventricle. The triangle found between the right and left coronary leaflets is composed of muscular tissue, the triangle between the left coronary and the noncoronary leaflets is a fibrous sheet in continuity with the anterior mitral valve leaflet, and the triangle between the noncoronary and right coronary leaflets comprises the membranous septum. Perforation of the last triangle will create a communication to the right ventricle (a ventricular septal defect), while the first two communicate directly with the pericardial space and perforation here will risk the development of cardiac tamponade. The anatomically weakest region of the muscular LVOT is the region between the left fibrous trigone and the left/right commissure.
Multislice Computed Tomography Assessment of the Aortic Annulus
Multi-slice computed tomography (MSCT) is the method of choice for pre-TAVI assessment of the aortic root and offers a comprehensive 3D image reconstruction of the anatomy. Accurate measurement of the annulus using MSCT is an integral part of pre-procedural planning and accurate transcatheter heart valve (THV)-sizing which is crucial to ensure valve anchoring, sealing and function. Integration of MSCT for THV valve sizing has been shown to reduce paravalvular leak (PVL) compared with 2D echocardiography.10–12
As the aortic valve apparatus undergoes dynamic change during systole and diastole, annular measurements vary during the cardiac cycle (with larger dimensions in systole).13–15 Area-based sizing is recommended for balloon-expandable THV; the goal is to oversize the THV relative to the annulus by 0–10 %.16 In contrast, self-expandable valves use perimeter (circumference) based sizing and oversizing of THV relative to the anatomy of 10–25 % is usually recommended, depending on the specific valve system.16,17 Excessive oversizing, particularly of balloon-expandable THV, is closely linked to annular rupture.18
In addition to annulus and root measurements, MSCT allows assessment of aortic root calcification. Koos, et al. validated quantification of aortic valve calcification by MSCT, using the Agatston AVC score, and demonstrated close correlation with in vitro calcification mass determined by atomic absorption spectroscopy.19. As discussed later in this article, some patterns of calcification predispose patients to annular rupture.
Incidence
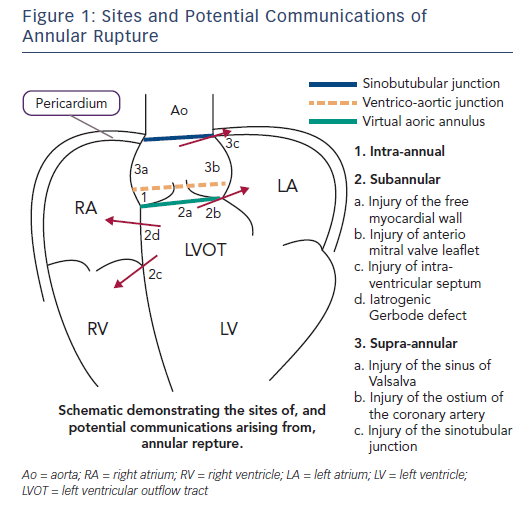
Aortic annular rupture is an umbrella term used to describe a variety of injuries that may occur in the aortic root and LVOT during TAVI. Figure 1 shows the potential locations for aortic annular rupture. Risk factors for rupture, clinical sequelae and management depend on the site involved. The incidence of annular rupture is reported as between 0.4 % and 2.3 %: increasing operator experience and the use of MSCT have seen a considerable decline in the frequency of this complication.20–24
Predictors of Annular Rupture
Anatomical
Several studies have demonstrated that subannular calcification predisposes patients to annular rupture,25,26 Barbanti et al. described an 11–fold increased risk of rupture with balloon-expandable THV in the presence of moderate to severe LVOT/subannular calcification (odds ratio 10.92; 95 % CI: 3.23–36.91; P<0.001).25 In another study, MSCT analysis of aortic root/LVOT suggested subannular calcification in close proximity to the region of the muscular LVOT between the left fibrous trigone and the left/right commissure in 83 % of patients with subannular perforation.26
A higher burden of LVOT calcification, especially when extending into the LVOT in the non-coronary cusp, has also been associated with aortic root injury during TAVI with balloon-expandable valves.27
Moderate to severe annular calcification is also an independent predictor of ≥mild PVL.28 Although not directly associated with annular rupture, annular calcification with ≥mild PVL usually prompts balloon postdilatation. Excessive or aggressive postdilatation is another risk factor for annular rupture.25
The risk of vascular complications (including annular rupture) is increased in patients with a smaller body surface area.24 Interestingly, even after correcting for smaller body size and weight, women have been have smaller aortic root dimensions than men (mean annulus diameter: 22.9 ± 2.2 mm versus 25.7 ± 2.7 mm; mean sinus of Valsalva diameter: 31.8 ± 4.2 mm versus 36.3 ± 3.8 mm; mean STJ diameter: 26.3 ± 3.4mm versus 29.8 ± 4.2mm).54 This information explains the overrepresentation of women (74 %) in a series of aortic annular ruptures with balloon expandable valves.25
Prosthesis Choice/Sizing
Several THV are used routinely in clinical practice. They include balloon-, self-, and mechanical-expandable systems (the Boston Scientific Lotus valve has been temporarily withdrawn from the market). The incidence of annular rupture depends on the type of prosthesis used and, indeed, balloon-expandable valves (SAPIEN XT/3, Edwards Lifesciences) are associated with much higher rates of annular rupture than self-expandable valves.18,25,29–31
With balloon expandable prostheses, the strongest predictor of annular rupture is MSCT-based area oversizing >20 %.18 Important work by Barbanti et al. found 37 consecutive patients undergoing balloon-expandable TAVI and experiencing root rupture had a greater degree of area-based prosthesis oversizing (30.5 % ± 15.8 % versus 11.3 % ± 19.7 %, p<0.001) and a higher frequency of post-dilatation (22.6 % versus 0.0 %, p=0.005) than those who did not experience this complication.25 This study determined that with balloon-expandable valves, MSCT-based area oversizing ≥20 % predicted an eightfold increased risk of rupture (odds ratio: 8.38; 95 % CI: 2.67–26.33; p<0.001).
As such, in patients at high risk of rupture, self-expanding or mechanical expanding valves may be preferable to balloon expandable valves. These prostheses are rarely associated with annular rupture unless excessive balloon pre- or post-dilatation is performed. An alternative technique to reduce the risk of annular rupture in the presence of adverse anatomical features with balloon expandable valves is underfilling of the deployment balloon (by 1–3 ml). This strategy and the now routine slow two-step deployment of the prosthesis have gained popularity, particularly in centres where self-expanding technology is not readily available.
Procedural technique
A few simple procedural techniques can reduce the risk of annular rupture. In particular, a comprehensive understanding of the aortic root anatomy from detailed MSCT can guide valve and balloon size selection. It is of utmost importance to respect the patient’s native anatomy: pre- and post-dilatation balloons should not exceed the mean diameter of the LVOT or sinotubular junction, whichever is smaller. It is recommended to use a balloon to artery ratio of 1.0 for semi-compliant balloons and <1.0 for non-compliant balloons. Failure to respect these “rules” increases the risk of annular rupture.25
Moreover, in patients with severely calcified annular or subannular anatomy, there has been a move towards undersizing the THV relative to the annular dimensions. In such cases, supra-annular sizing is performed using a variety of dedicated techniques and, although the THV is undersized relative to the annulus, it is not undersized relative to the supra-annular structures. This technique is particularly useful and increasingly used in cases of bicuspid aortic valve stenosis, where the presence of a raphe (Siever’s type 1 or type 2) or dense leaflet calcification permits relative “downsizing” of the valve. For balloon-expandable THV, this usually manifests as an underfilling of the deployment balloon by 1-3 cc. This technique and further post-dilatation, if required, have been shown to reduce the risk of annular rupture without increasing PVL.34 With self-expanding THV, the size of the pre- and post-dilatation balloons and the THV itself may be reduced. To the authors’ knowledge, annular rupture in the absence of post-dilation has not been reported with the self-expanding technology.
Management of Aortic Annular Rupture
Diagnosis
The clinical manifestations of annular rupture can vary, and depend on the extent and site of the disruption. Rapid haemodynamic collapse is the most common presentation and is due to rupture into the pericardial space and ensuing cardiac tamponade. Ventricular septal defect, fistulae from the left ventricle to the atria (iatrogenic Gerbode defect) and perforation of the anterior mitral valve curtain may be better tolerated acutely, while localised perforation with intramural haematoma can present insidiously or may only be identified on post-TAVI imaging.35–37.
Hypotension or haemodynamic instability should always arouse suspicion of annular rupture. Haemodynamic instability may manifest as increased central venous pressure, low arterial blood pressure, tachycardia, arrhythmia or frank circulatory collapse with a combination of these features. Patients may report pain due to pericardial irritation. Immediate angiography and echocardiography are the diagnostic modalities of choice. Any evidence of bleeding into the pericardium should arouse suspicion of annular rupture. Serial imaging and examination may be required to confirm or unequivocally exclude the diagnosis.
Initial Approaches
In patients without haemodynamic compromise, such as those with self-contained or limited rupture or aortic root haematoma, a conservative management strategy can be adopted after a thorough imaging assessment. In such cases, monitoring the haemodynamic status and reversal of systemic anticoagulation may suffice. Nevertheless, contingency plans and escalation strategies should be prepared, including an immediate heart team discussion. In stable patients, reversal of anticoagulation, ensuring the availability of blood products and frequent reassessment of clinical status should be considered. Transoesophageal echocardiography and MSCT can help define the extent of the aortic root disruption.36
Contained rupture usually has a favourable outcome. These events occur more frequently than originally thought; they were identified in 1.2 % of patients after a balloon expandable TAVI in a large multicentre report with systematic computed tomography angiography (CTA) performed after TAVI.39 Subacute progression of the rupture can occur, and late development of aortic pseudo-aneurysm has been reported.53
In patients with haemodynamic collapse, associated pericardial effusion and cardiac tamponade, percutaneous pericardial drainage and reversal of systemic anticoagulation should be performed. Not infrequently, these manoeuvres may be sufficient to stabilise the patient, but contingency plans for escalation should be instituted on confirmation of the diagnosis.
Auto-transfusion of the pericardial aspirate can reduce the need for blood products when bleeding is extensive. Haemodynamic support or extracorporeal membraneous oxygenation can provide a bridge to surgical repair. When pericardial bleeding cannot be controlled, sternotomy, initiation of cardiopulmonary bypass, and aortic root repair with or without surgical aortic valve replacement is required.
In the cases of patients who are not suitable for surgical intervention, recombinant factor VIIa has been reported to reduce bleeding, though the efficacy of this strategy remains controversial.41
If the rupture site can clearly be identified as either immediately cranial or caudal to the skirt of the THV, then implantation of a second THV to seal the rupture has been successfully performed.46 In such cases, the second valve is intentionally positioned either cranial or caudal to the initial implant, according to the site of rupture. This has primarily been described in balloon expandable valves.42 In one case, pre-dilation with a 23 mm balloon followed by a 26 mm Edwards valve was performed. Angiography demonstrated an annular tear and cardiac tamponade was demonstrated on echocardiography. Despite pericardiocentesis, auto transfusion and attempted sealing of the annular tear with another valve, the patient died.46 The authors of the study noted that two-thirds of the patients in their series underwent post-dilatation with relatively large balloons. They proposed an initial strategy of post-dilatation with a smaller balloon and to proceed further with a larger balloon if necessary. They also proposed choosing a smaller valve size for patients with heavy annular calcification.
Surgical options
The development of emergency algorithms to manage life-threatening complications such as annular rupture with TAVI is axiomatic. Clear pathways and protocols need to be designed to streamline care, including expedited transfer to surgical theatre if a hybrid lab is not available.
Intubation, ventilation and emergency sternotomy with exploration of the aortic root to identify and treat the source of bleeding should be considered when persistent uncontrollable haemorrhage occurs. Cardiopulmonary bypass (CPB) maybe required to help stabilise haemodynamics. This can usually be instituted as normothermic femoro-femoral cardiopulmonary bypass. Central cardiopulmonary bypass through direct aortic cannulation is an alternative option and may be used in patients with severe peripheral arterial disease.
The type of surgical treatment depends on the origin and extent of annular rupture. The standard surgical operation in cases of intra-annular rupture comprises of removal of the TAVI prosthesis, excision of the native aortic valve, repair of the ruptured annular lesion with an autologous pericardial patch and aortic valve replacement with a prosthetic valve.31,35
The treatment for supra-annular rupture depends on the site involved. Injury of the coronary ostium can be treated with composite valved graft or aortic valve replacement plus repair of the rupture site. Injuries of the wall of sinus of Valsalva or of the sinotubular junction require repair of the lesion using a pericardial patch or pledgeted sutures and aortic valve replacement. Subannular injury of the interventricular septum or free myocardial wall requires repair or reconstruction using a pericardial or synthetic patch by a transaortic approach, in addition to aortic valve replacement.42–45
Traumatic ventricular septal defects (VSDs) may be well tolerated or, conversely, be associated with subacute or acute haemodynamic compromise. Either a surgical or percutaneous strategy can be effective in such situations depending on the extent and location of the defect, stability of the patient and experience of the institutional heartteam.46 A recent systematic review of iatrogenic VSD following TAVI identified 20 patients from 18 case reports. Of these 20 patients, 13 (65 %) were managed conservatively and seven (35 %) required defect closure.48 Among those requiring intervention, percutaneous techniques were employed in six and one patient underwent surgery. Devices used for closure in these cases included the Amplatzer septal occluder, Amplatzer muscular septal occluder, Amplatzer VSD occluder and the Amplatzer muscular VSD occluder (St Jude Medical). A retrograde technique was used for closure in three cases. While concerns have been expressed regarding the effect of transcatheter VSD closure on TAVR function, no issues with valve function or valve dislodgement were reported in these case reports. Four out of 20 (20 %) patients died in hospital, all of whom were managed conservatively. The reports proposed that risks for VSD after TAVR included: severe asymmetric calcification of the valve; elliptic aortic annulus; valve oversizing; and higher valve placement. The authors also noted that many patients in the study were managed successfully without intervention and proposed that VSD closure should be reserved for patients who had failed conservative management.
Outcomes
Annular rupture can be a catastrophic complication of TAVI. Although mortality varies between studies, high 30-day mortality rates of 49–67 % have been reported. Annular rupture is associated with a seven-fold increase in 30-day mortality. In cases of contained rupture, mortality is lower (~25 %) but nonetheless remains considerable.22,49
Among patients with a rupture proceeding to emergent surgery, specific mortality data is scarce. However, conversion from TAVI to an open surgery has been associated with a 30-day mortality rate of 45 %, regardless of aetiology.45 In a recent study by Eggebrecht et al. on outcomes of patients undergoing emergent cardiac surgery as a result of life-threatening complications during TAVI, annular rupture accounted for 21.2 % of all cases.50 In-hospital mortality for annular rupture was 62 %, which compared poorly with that for the overall population (46 %).
Future Directions
As TAVI is offered to younger and lower-risk patients, it is paramount that practitioners remain vigilant in preventing serious complication such as annular rupture. Data on the incidence of annular rupture among younger patients are sparse.
In the NOTION 1 trial of intermediate-risk patients treated with first generation TAVI devices, conversion to surgery was required in 2.1 %.51 Importantly, the incidence of bicuspid aortic valve and hence severe valvular calcification is greater in younger patients. It is therefore possible that rates of annular injury could be higher in this patient group. Retrospective registry data has previously reported aortic root rupture rates of 0.7 % in TAVI for bicuspid aortic valves.52 However, there is little prospective data on this topic and further research is needed. Tailored THV sizing strategies in bicuspid aortic valve morphology should mitigate the risk of rupture in these patients.
Conclusions
Annular rupture is a rare but serious complication of TAVI. Pre-procedural MSCT screening is essential to recognise potential predictors of annular rupture, especially subannular calcification, and to appropriately size the THV. Balloon expandable valves and aggressive balloon dilatation should be avoided in such cases.
Prompt management of rupture includes haemodynamic support, reversal of anticoagulation, pericardial drain, consideration of a second THV and emergency surgery. A dedicated protocol should be developed by the institutional heart team for such emergencies.