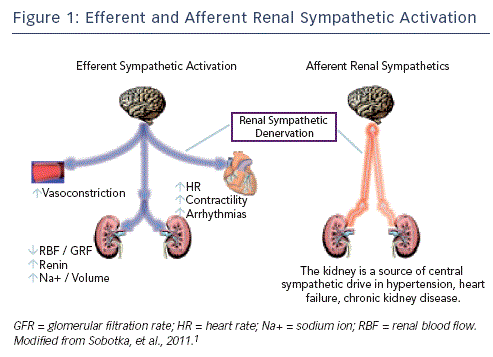
The sympathetic nervous system regulates cardiac output, blood pressure (BP), heart rate and volume, electrolyte balance and composition of body fluids. The afferent signals to the brain are regulated by mechanosensitive baroreceptors, volume sensors and chemoreceptors located in the vessels and in many other organs, particularly in the heart, kidney, lung, brain and muscles. Sympathetic nervous system outflow is coordinated by the nucleus tractus solitarius, located in the midbrain.1 Efferent sympathetic signals regulate BP, heart rate and cardiac output through beta (β)-adrenergic receptors, and regulate peripheral resistance by vasoconstriction through alpha (α)-adrenergic receptors. In the kidney, sodium and water retention is stimulated by activation of β-adrenergic receptors in the proximal tubules.1
Renal Sympathetic Nerves
Renal sympathetic nerves regulate kidney function, volume homeostasis, cardiac output and BP control.2 Renal sympathetic activation results in volume retention, sodium reabsorption, reduction of blood flow and renin-angiotensin-aldosterone system activation.2 However, the kidney also has an extensive network of afferent unmyelinated fibres that transmit important sensory information to the central nervous system.2,3 Afferent fibres from the kidney have been shown to travel along with the sympathetic nerves at the level of the kidney and then enter the dorsal roots and project to neurons at both spinal and supraspinal levels. Most of the brainstem regions involved in cardiovascular control including the hypothalamus receive inputs from the renal afferents, which carry information to the central nervous system from renal chemo- and mechano-receptors.2,3 Direct electrical stimulation of the renal afferent nerves in animals can produce both sympathoinhibitory and sympathoexcitatory reflexes, illustrating the diverse functional nature of various populations of renal receptors.3
In Figure 1, the interaction between efferent and afferent sympathetic activation and the kidney are shown. Activation of renal efferent sympathetic nerve fibres increases sodium and water retention, reduces renal blood flow and elevates renin release from juxtaglomerular apparatus, regulating BP. Renal nerves contain sensory afferent fibres, which enable communication with the central nervous system. Activation of renal afferent nerves itself elevate sympathetic nervous outflow to the kidney and other downstream organs (see Figure 1).
Renal Sympathetic Denervation
Recently, a catheter-based approach has been developed for renal sympathetic denervation (RDN). RDN significantly lowers BP in patients with therapy-resistant hypertension. Both, a proof-of-concept trial (Symplicity HTN-14), including 50 patients and a multicentre, prospective, randomised trial (Symplicity HTN-25) including 106 patients with resistant hypertension (systolic BP ≥160 millimetres of mercury [mmHg], >150 mmHg for type 2 diabetes) despite receiving ≥3 antihypertensive drugs) demonstrated that RDN significantly lowered BP. Systolic BP in these clinical trials was reduced by 25–32 mmHg. Three hundred and forty-six uncontrolled hypertensive patients, separated according to daytime ambulatory BP monitoring into 303 with true resistant (office systolic BP 172 mmHg; 24-hour systolic BP 154 mmHg) and 43 with pseudoresistant hypertension (office systolic BP 161 mmHg; 24-hour systolic BP 121 mmHg), were analysed in another study.6 At three, six and 12 months follow-up, office systolic BP was reduced by 21/23/27 mmHg and office diastolic BP by 8/9/11 mmHg, respectively. In patients with true treatment resistance there was a significant reduction with RDN in 24-hour systolic BP (-10/-10/-11 mmHg), diastolic BP (-4/-4/-7 mmHg), maximum systolic BP (-11/-10/-6 mmHg) and minimum systolic BP (-6/-9/-13 mmHg) at three, six and 12 months, respectively. There was no effect on ambulatory BP monitoring in pseudoresistant patients, whereas office BP was reduced to a similar extent.6
A multicentre, prospective SYMPLICITY HTN-3 study, which completed enrollment, will evaluate the safety and efficacy (including 24-hour BP measurements) of RDN in approximately 600 patients with resistant hypertension.7 In contrast to the Symplicity HTN-1 and HTN-2 trials, SYMPLICITY HTN-3 is a single-blind study with one group undergoing a sham procedure. This design will hopefully address the question of whether placebo-like effects contribute to the observed outcome after RDN.
Mechanistically, it has been observed that the procedure resulted in a 47 % reduction of renal norepinephrine spillover measured with a radiochemical tracer methodology using 3H-norepinephrine.4,5 Interestingly, firing of single sympathetic vasoconstrictor fibres, measured by single muscle sympathetic nerve activity were reduced by 37 %.8 These findings indicate a combined modulation of efferent and afferent signalling at the kidney by RDN.
Patient Selection
Patient selection criteria should be consistent with those used in the clinical trials, in which eligible patients with resistant hypertension had an office systolic BP ≥160 mmHg (≥150 mmHg in patients with type 2 diabetes), despite treatment with at least three antihypertensive agents, including one diuretic, in adequate doses.9 Single-centre experience has been reported for interventional RDN in patients with mild hypertension.10 However, RDN cannot currently be recommended for the treatment of mild hypertension, or for prophylaxis of hypertension, outside clinical trials.10 Secondary hypertension should be excluded (except for obstructive sleep apnoea syndrome) and patient compliance according to medical treatment should be identified. To define which patients are suitable (diameter >4 millimetres [mm]; length >20 mm) for the intervention, renal artery anatomy must be investigated by duplex ultrasonography or magnetic resonance imaging (MRI)/computed tomography (CT) scan and renal function tests (glomerular filtration rate [GFR] >45 millilitres per minute [ml/min] per 1.73 square metres [m²]) should be performed to reduce the risk of a decline in renal function following procedural exposure to contrast dye. RDN cannot currently be recommended for the treatment of hypertension in patients with chronic kidney disease outside clinical trials.11,12
Procedure Itself
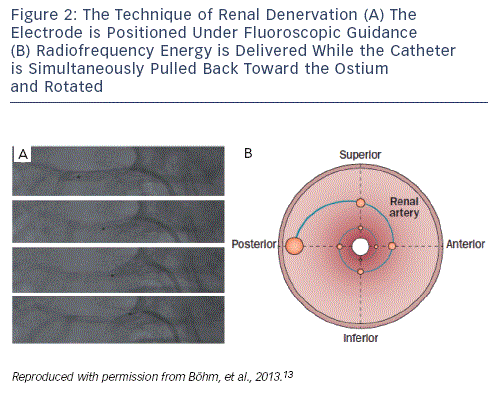
There are currently six CE-mark renal denervation systems commercially available. The largest experience, although still limited in numbers, exists for the Symplicity system (Medtronic, US). First-generation devices use radiofrequency pulses emitted from a monopolar electrode positioned under fluoroscopic guidance in each of the renal arteries. At least four ablation points per artery are recommended. The catheter is positioned at the periphery of the second order renal artery branch point. Energy (maximum 8 watts [W]) is delivered with an impedance of 250–320 ohm (Ω) to the ablation points. During energy delivery, the catheter is rotated and pulled back to the ostium of the renal artery, to treat the total circumference of the renal artery (see Figure 2). The distance between successive ablation points is recommended to be ≥5 mm. Operators should be experienced in handling complications, such as renal artery dissection or perforation. Since group C nerve fibres, which carry pain signals, run alongside the sympathetic nerve fibres, the procedure is painful and requires sedation and local analgesia. High-frequency energy delivery also generates heat in the vessel walls. Despite being internally cooled by the high rate of blood flow, heat accumulates in the adventitia of renal arteries where sympathetic nerves are located.13
Non-responder
The BP response after RDN differs. Only minor or no changes in BP occur in 8–37 % of patients.14 The reasons for non-response (defined as a reduction in office systolic BP of <10 mmHg six months after the procedure) are not completely understood.
Additional Effects of Renal Sympathetic Denervation
Reduction of sympathetic nervous system by RDN might also show beneficial effects by influencing cardiac arrhythmias,15 diabetes and metabolic syndrome,16 kidney disease17 and congestive heart failure.18 However, the definite role and safety of RDN in these patients needs to be investigated in bigger clinical trials.
Summary
Renal sympathetic denervation reduced BP in resistant hypertensive patients. Further technical refinements and the practical testing of new devices might increase efficacy and safety of the procedure. Beneficial additional effects of RDN might be useful to treat a population of patients with a range of diseases.