Mark Anderson was previously the Chair of the Cardiothoracic Surgery at Einstein Medical Center in Philadelphia. Dr Anderson specialises in the surgical management of heart failure and myocardial recovery.
Dr Anderson gave a surgeon’s perspective on cardiac unloading. He commenced by reminding the congress that cardiopulmonary bypass is the foundation of cardiac unloading. By placing the patient on bypass, the heart is completely unloaded, allowing the surgeon to conduct the necessary procedure. However, while more patients are surviving following acute myocardial infarction (AMI), evidence indicates that heart function is not being necessarily recovered. This increased survival rate with insufficient heart recovery is leading to more hospital admissions and an increased rate of heart failure.
Mechanical circulatory support (MCS) is increasingly being recognised as a valuable intervention in AMI. The US Food and Drug Administration states that: “The Impella 2.5, Impella CP, Impella 5.0 and Impella LD catheters, in conjunction with the Automated Impella Controller console, are intended for short-term use (≤4 days for the Impella 2.5 and Impella CP and ≤6 days for the Impella 5.0 and Impella LD) and indicated for the treatment of ongoing cardiogenic shock (CS) that occurs immediately (<48 hours) following AMI or open heart surgery as a result of isolated left ventricular failure that is not responsive to optimal medical management and conventional treatment measures with or without an intra-aortic balloon pump. The intent of the Impella system therapy is to reduced ventricular work and to provide support necessary to allow heart recovery and early assessment of residual myocardial function.” This statement emphasised the potential for the Impella® (Abiomed) pump in heart recovery and established a role for surgery in MCS.
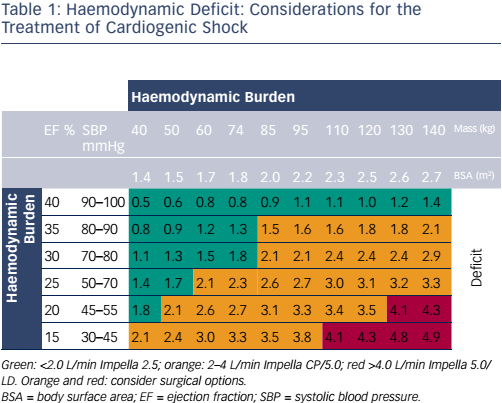
Following the availability of these powerful new interventions, clinicians need guidance on how to optimise their use. Table 1 shows the factors that should be considered when choosing the level of support in haemodynamic deficit and when surgery is needed. Another tool that can help in clinical decision-making is cardiac power output, a potent indicator of mortality.1 Interventions can be targeted on the basis of early cardiac power output and subsequently assessed. Full unloading can optimise recovery, but there is a need to meet the demands for increased unloading and support. The early decision to both initiate and escalate MCS is particularly important in optimising outcomes. The Impella 5.0 is the most commonly used device for escalation in current surgical clinical practice.
There is also a need for more clinical evidence to guide the use of these devices. Since large randomised clinical trials involving cardiogenic shock patients are difficult to conduct, the global catheter-based Ventricular Assist Device Registry™ has been created.2 Its purpose is to capture data reflecting real-world use of Impella devices in current clinical practice and provide insights into patient characteristics, co-morbid conditions, outcomes, patterns of care and the performance metrics of participating institutions to guide improvement in ventricular assist device use. Data from the registry show that surgical devices are still needed in advanced cases. Percutaneous technology is still associated with disadvantages, including instability of femoral artery placement and the restriction of the patient being confined to bed to recover. Axillary artery implantations allow for patient mobility and a more rapid patient recovery.
The use of ventricular assist devices in AMI complicated by cardiogenic shock has a number of advantages: it completely rests the heart, reduces the need for inotrope/pressor support and provides stability during acute events. It is an effective bridging strategy and an optimal recovery platform. In order to optimise its use in routine clinical practice, there is a need for collaboration between interventional cardiologists and surgeons.
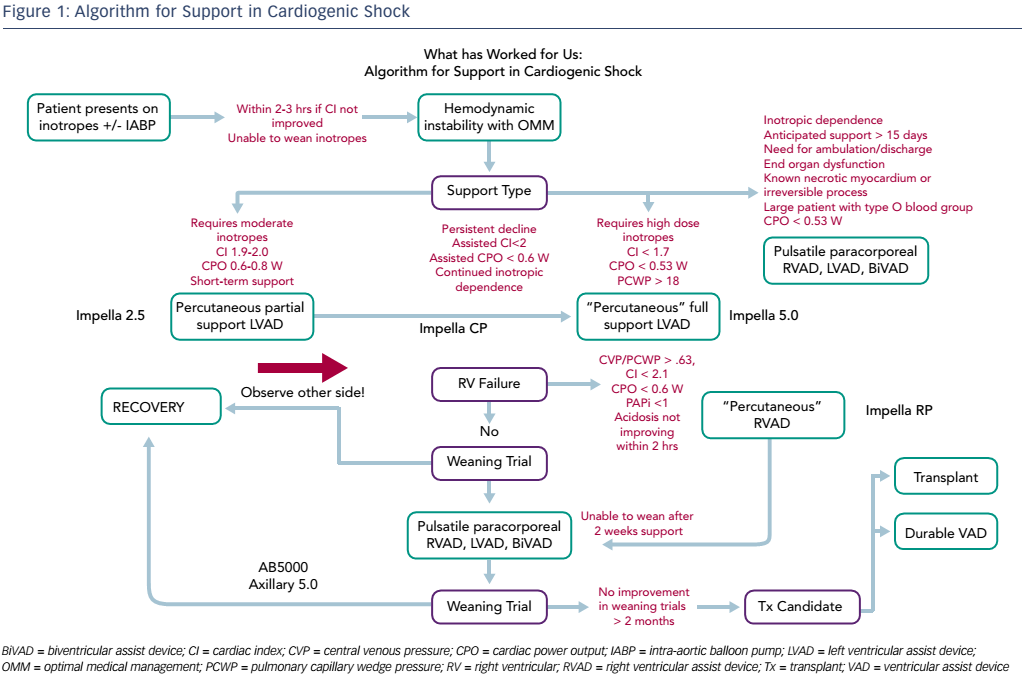
Dr Anderson finished by highlighting the need for a standard treatment algorithm and presented an algorithm that has proven effective in his centre (see Figure 1). He concluded that this meeting has demonstrated some important paradigm shifts in the management of cardiogenic shock, from partial unloading to optimal unloading; from later referral to early escalation; univentricular MCS to biventricular MCS; and the concept of a bridge to recovery rather than to a transplant.