Chronic total occlusions (CTO) are a common finding at angiography in patients with coronary artery disease (CAD); they are present in ~20 % of cases at angiography (excluding those with acute MI or prior coronary artery bypass graft (CABG).1 Data from the same Canadian registry showed that only 10 % of CTO patients had had a percutaneous coronary intervention (PCI) attempted to treat CTO, with only 7 % successfully revascularised by this procedure. Recent data continue to demonstrate a low rate of CTO PCI in the UK, with approximately 5 % of all PCI lesions being a CTO procedure and ~12–13 % of PCI for stable lesions being a CTO PCI2.
The presence of a CTO has historically led patients to be referred for a CABG or a recommendation for medical therapy. Rates of CTO PCI most probably continue to be low because of the perception that these lesions are technically challenging to deal with, and that the procedure has low success rates and higher complication rates. This is despite a large body of evidence demonstrating that high procedural success rates (of ~90 %) are now routine across large groups of patients in different geographies, as well as among operators and centres with varying experience.3–5
Rates of adverse events associated with CTO PCI
Traditionally, PCI for non-occlusive disease is viewed as a low-risk procedure for patients with stable CAD. Rates of early Q-wave MI, emergency CABG, cerebrovascular accident (CVA) and mortality are reported at 0.03 %, 0.02 %, 0.04 % and 0.14 %, respectively, in a national audit of >100,000 PCIs annually.2
However, as with all registries, the potential for under-reporting complications (especially events that occur several hours after the PCI) should always be borne in mind. For example. in recent large PCI studies, the in-hospital major adverse cardiac events (MACE) rates associated with left main PCI were noted to be ~4 %6 and 5 %7 when there were systematic follow-up and clinical events committees for adjudication. This number is well in excess of the ~0.2 % MACE rate (death, stroke or Q-wave MI) for PCI for stable CAD and non-occlusive disease noted in the UK audit.
Rates of inpatient mortality and MACE from a single-centre series of 25 years of CTO PCI has, reassuringly, suggested success rates are increasing while adverse events are declining over time.8 Inpatient adverse events were described among 18,061 patients and 18,941 target CTO vessels in a meta-analysis.9 These data suggest low risks for mortality (0.2 %), emergent coronary bypass graft (0.1 %), stroke (0.01 %) and contrast nephropathy (3.8 %).9 Perforation was reported at 2.0 % with cardiac tamponade in 0.3 %. The approach taken can also influence adverse events, with retrograde procedures being associated with higher numbers of these outcomes.10 While mortality remains low (0.7 %), collateral perforation (6.9 %) and tamponade (1.4 %) were noted amongst 3,482 patients undergoing retrograde CTO PCI procedures.
As discussed above, the accuracy of these data (that are almost all registry based) may be limited and, as such, they should be interpreted cautiously. The OPEN CTO registry has been reported recently. In contrast to previous reports of CTO PCI outcome, this study was linked to the National Cardiovascular Data Registry registry to ensure that all consecutive patients were assessed. Furthermore, angiographic core laboratory and central clinical event adjudication were applied for all patients. Under these circumstances of systematic and robust clinical follow-up for contemporary CTO PCI, adverse event rates were noted to be more frequent.5 In-hospital mortality was found to be 0.9 % and a coronary perforation requiring intervention was 4.8 %. Modern CTO PCI techniques do afford intervention for much more complex disease and this is likely to be the main factor in an upturn in complications. Nevertheless, these data should be viewed as being derived from a gold standard for outcome reporting. Where outcome data are reported without this level of robust follow-up, discernment with regards to their absolute validity is required.
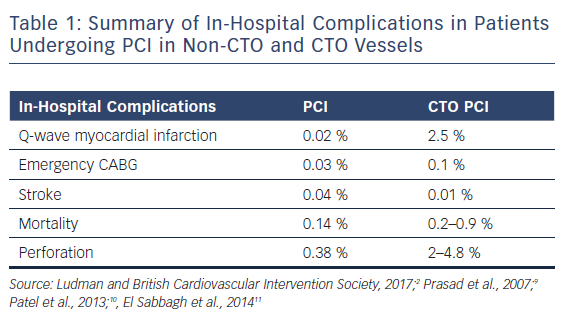
Table 1 gives a summary of in-hospital complications in patients undergoing PCI in non-CTO and CTO vessels.
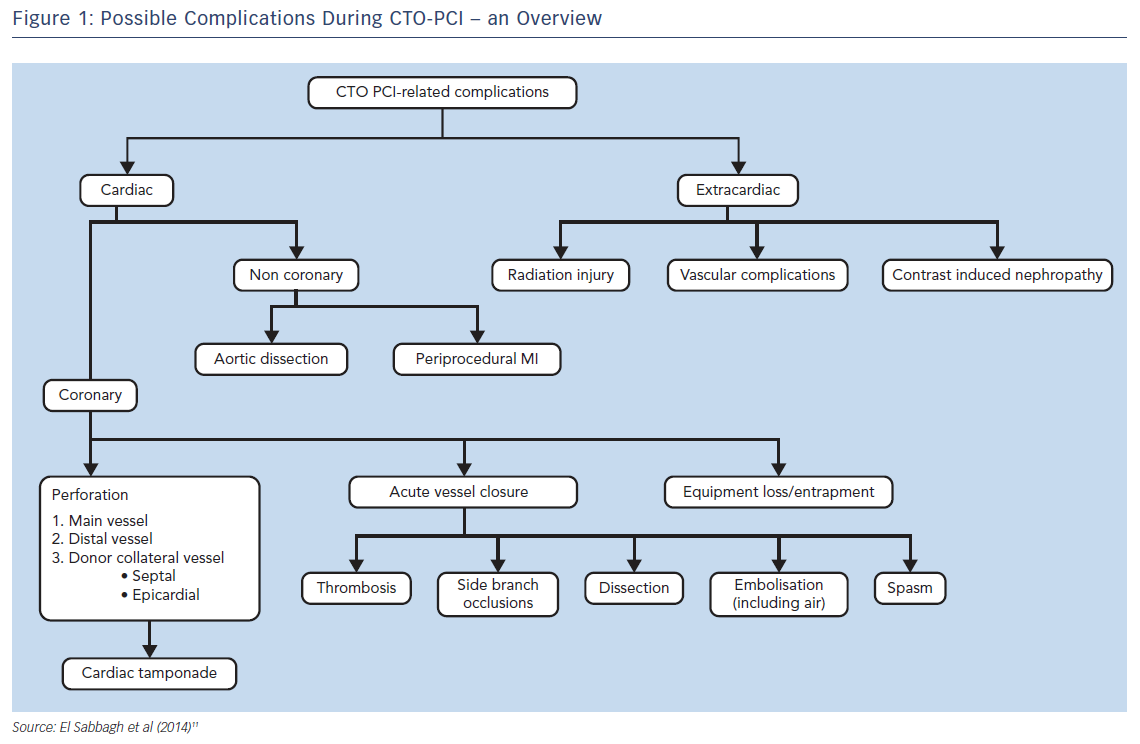
Regardless of the small absolute numbers of serious complications, all operators should be aware of these events and should be able to treat them immediately if needed. CTO-related complications can be considered as acute or late as well as cardiac (coronary and non-coronary) or extracardiac complications (Figure 1).11
Specific risks in CTO PCI include: acute myocardial infarction due to compromise of collateral flow; coronary perforation with pericardial blood extravasation with or without cardiac tamponade; damage of the proximal occluded segment due to deep guide catheter intubation; donor vessel damage (mechanical or thrombotic) during retrograde crossing; and renal failure due to excessive contrast load.11
Perforation and Collateral Channel Injury
Coronary perforation is a well-known complication of CTO PCI. Left undiagnosed or untreated, this may become life threatening. The commonest scenario leading to perforation is of a guide wire exiting from the CTO body during attempted antegrade wire escalation (AWE). As long as the wire is not followed by secondary equipment, this is typically a relatively forgiving scenario without significant clinical sequelae.
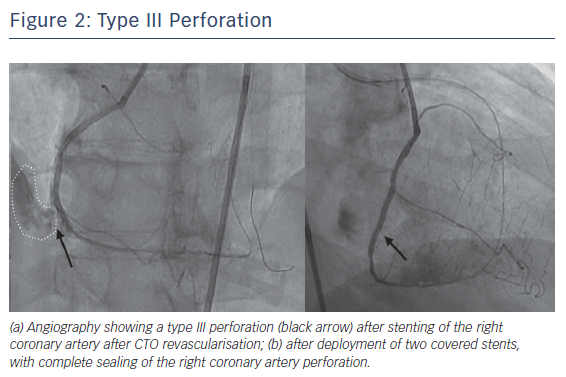
Perforations leading to significant bleeding are unusual, but those requiring an intervention (~1–2 % in registries and almost 5 % in OPEN CTO5) are common enough to require familiarity with corrective manoeuvres. In the authors’ experience, those associated with tamponade are most commonly due to collateral channel injury during a retrograde procedure or from unrecognised distal wire exits after antegrade crossing. Other potential causes include passing equipment out of small branches because the operator does not realise they are not in the main vessel architecture, or where stent deployment (or postdilation) leads to contrast extravasation as would also be seen in routine PCI (Figure 2).
Should a significant perforation occur, the first step is to prevent continuing blood loss. This is usually achieved by inflating a balloon (1:1 sizing) in the proximal portion of the leaking vessel. If there is a significant bleed while working with a 6F or 7F guide catheter, it is often useful to site a second guide catheter in the same vessel (a “ping-pong guide”). This will allow the operator to maintain control of the bleed, while briefly deflating the occlusion balloon, then reinflating it once equipment has passed distally to facilitate distal intervention. However, a ping-pong guide is not required if a 8 F guide is used when covered stent delivery and continued balloon inflation can be managed via a single guide. Operators should be aware that perforations from epicardial collaterals can be “fed” from multiple sources and that control of continuing bleeding can be challenging.
The next question is whether there is haemodynamic compromise. If the patient has hypotension, an echocardiogram can confirm a diagnosis of cardiac tamponade and aid subsequent pericardiocentesis. With this in mind, it is important that catheter laboratories that perform regular CTO PCI have this equipment to hand with immediate availability in case it is needed for emergent use.
Subsequently, the operator needs to identify the exact source of bleeding and the steps that are most appropriate to prevent ongoing blood loss. A specific inventory is necessary to be able to resolve the underlying abnormality. Potential solutions include the injection of autologous fat through a microcatheter (often effective for distal branch wire exits), coils (frequently required for collateral channel perforation), micro-beads, thrombin or covered stents. An appropriate range of this equipment must be available at all CTO PCI centres.
Some specific scenarios can arise with collateral channel injury during retrograde approaches that require recognition and different steps for resolution. When there has been exit into a cardiac chamber, these events are almost always benign and self-limiting. It is important that the nature of any ongoing extravasation is understood. Fat, thrombin or micro-beads should never be injected if there is a collateral leak into a left heart chamber as these can potentially embolise and cause a stroke. Very large septal haematomas have also been described, including those that lead to a “dry tamponade”. Occasionally, these will spontaneously decompress12 by forming a self-limiting ventricular septal defect. Where a very large septal haematoma leads to patient compromise, this can be decompressed by percutaneous intervention. Under these circumstances, if the operator can pass a wire from the left coronary side via the haematoma cavity to the right ventricle, passage of a micro-catheter or 2 mm balloon to dilate this connection may allow the haematoma to drain without further compromise to the patient.
Other potential sequelae of collateral channel exit are localised pressure effects, which are seen most often in the patients who have had a CABG. It is not unusual to see right ventricle free wall haematomas form when there has been over-aggressive dissection in the right coronary artery or when there has been dissection into branches with or without equipment exit in a “closed-chest” patient. Typically, supportive measures with intravenous fluids and/or inotropes will be sufficient to support the patient and allow spontaneous resolution. In the post-CABG setting, leaks from atrioventricular groove epicardial collaterals can lead to a left atrial pseudo myxoma and impaired mitral inflow.13 These are often best diagnosed by CT angiography and may require localised drainage using CT guidance13 or open surgery in rare circumstances.
Where retrograde approaches are considered, interventional cardiologists should be mindful of the hierarchical risk associated with each approach. In general, the use of patent (but diseased) vein grafts is safe. Septal collaterals are usually forgiving, and minor perforations tend to be well tolerated. Epicardial channels are less forgiving should a complication occur and should be considered as the last option. The authors would advocate avoiding the use of a left internal mammary artery (especially as a last remaining conduit) where at all possible.
Finally, cardiac surgery may be required as a bailout option for percutaneously unresolvable bleeding. Centres that do not have on-site cardiac surgery facilities should strongly consider whether the use of epicardial collaterals and certain techniques is appropriate. In addition, clear pathways should be established for the transfer of patients to surgical centres under emergent circumstances should the need arise.
CTO Strategies, Vascular Access, Radiation Injury and Contrast Nephropathy
A number of strategies can be adopted for CTO PCI. Many lesions are amenable to two or more approaches. An algorithmic approach to CTO PCI was first suggested in 2012; this recommends using the anatomy of the lesion to guide the initial strategy to approach the lesion.14 Four key questions guide the operator towards AWE, antegrade dissection and re-entry (ADR), retrograde wire escalation (RWE) or retrograde dissection and re-entry (RDR). An important aspect of a hybrid approach to CTO PCI is that operators must be able to recognise when they are entering a failure mode and efficiently switch between approaches. The main goal of this strategic switching is to minimise procedure duration, radiation exposure and contrast load. The use of the hybrid approach has been shown to be associated with high procedural success rates (~90 % per lesion) with acceptable use of contrast and radiation.3-5,15,16
Several basic steps can be taken to reduce radiation doses. These include reducing frame rates for both fluoroscopy and acquisition runs, minimising the use of acquisition runs to points in the case where they are both necessary and useful, and strongly encouraging operators to be vigilant around the use of X-rays. Where CTO PCIs are performed in institutions with multiple x-ray systems, performing these procedures with the most modern x-ray equipment is likely to reduce exposure.17
Contrast nephropathy should be considered as a potential adverse event related to CTO PCI. Minimising contrast use is key and, once again, efficient and appropriate use of CTO strategies is pertinent to prevent wasting contrast. However, several peri-procedural manoeuvres can reduce this risk. These include stopping nephrotoxic agents for 48 hours before the procedure (especially angiotensin-converting-enzyme [ACE] inhibitors or angiotensin II receptor blockers [ARBs]), pre-hydrating patients with intravenous fluids and/or the use of the RenalGuard system.
Historically, the use of certain strategies within PCI led to consideration of the use of 8F guiding systems (especially for ADR). Data have shown an association between sheath size and an increased risk for major bleeding19. Fortunately, the progressive evolution of CTO PCI dedicated technologies means that 8 F-guiding systems are rarely necessary now. Vascular complications related to femoral arterial access still occur in a significant number of cases in contemporary practice (0.3 % of patients required treatment in OPEN CTO, with haematomas noted in 4.3 %5; major vascular complications were noted in 2.5 % of RECHARGE patients4). The routine use of fluoroscopy to guide femoral puncture18 and/or the use of ultrasound may help to minimise adverse events. However, bilateral transradial access will obviate the risk of any femoral bleeding and is now feasible for the vast majority of CTO cases; it should be considered as an option where feasible and indicated.
Donor Vessel Issues
In general, if there is no retrograde approach there are limited implications from seating a guide catheter in the non-CTO (contralateral) vessel. Rarely, and often in the context of profound ischaemia with very severe left ventricular dysfunction, bilateral contrast injection can lead to ischaemia or arrhythmia. Under such circumstances, sequential single catheter injections may be safer.
Retrograde approaches raise numerous and specific issues related to the donor vessel. Any compromise to this system is potentially catastrophic, as the territory supplied is at risk as well as the CTO territory. Complications can lead to extensive ischaemia and rapid haemodynamic collapse and the operator should always consider this possibility if a patient becomes acutely unwell.
Donor-vessel occlusion due to thrombus formation should be avoided at all costs. While this complication can occur in the target vessel during antegrade CTO PCI, the risk is higher for retrograde access. The excess of intracoronary equipment is associated with stasis in the guide catheters. Therefore, meticulous attention must be paid to optimal anticoagulation using the activated clotting time (ACT) to minimise the risk for donor system thrombosis. After an initial bolus of 100 U/kg, the ACT should be monitored to maintain a level >300 seconds (some advocate 350 seconds) to minimise the risk. Back-bleeding of guide catheters and recurrent flushing is also recommended after material exchange.
Donor vessel dissection causing occlusion is relatively rare. This complication is most likely to occur as retrograde equipment is withdrawn from the CTO vessel. Under these circumstances, friction and energy that has been stored in the system during micro-catheter crossing is released. An equal and opposite reaction can occur as equipment is brought out of the CTO territory back to the donor guide and the guiding catheter can be sucked into the donor vessel. This is best avoided by withdrawing the donor guide catheter well away from the ostium of the artery as the equipment is being withdrawn, and continuously imaging to ensure that the catheter is not pulled deeply into the donor vessel. The authors would also advocate the simple step of leaving a workhorse guide wire in the distal segment of the donor artery throughout the procedure to facilitate bail-out intervention should a problem arise.
Occasionally, retrograde approaches can lead to kinking of the donor vessel, especially when the micro-catheter is advanced into a tortuous native vessel with significant angulation into the donor collateral. Under these circumstances, options are limited. If ischaemia and compromise occur, a braided micro catheter (Finecross, Caravel etc) that is more flexible and has a lower profile than a coil-based micro catheter (Corsair, Turnpike etc) may afford continued retrograde manoeuvres. However, if these devices are also associated with compromise of the donor vessel, then the retrograde approach may have to be altered (to an alternative collateral) or abandoned.
Equipment loss and entrapment are rare. Stents can come loose from their delivery balloon during attempted delivery through long, calcific and tortuous lesions. Low-threshold use of guide catheter extensions seems to obviate this risk in the majority of CTO PCI. Should this issue occur, retrieval is the same as during routine PCI.
Specific to retrograde CTO PCI, guide wires can become knotted after crossing into the CTO territory though this is rare and avoidable. This is a risk where knuckling has been performed, especially if the operator mistakenly “spins” the knuckle wire (this should be avoided). Pushing the retrograde equipment is more likely to free it up than pulling. Swapping the micro-catheter with a retrograde balloon and inflating this at the problem area may loosen any tissue that is entangled with the equipment. Similarly, siting antegrade equipment in the same area and ballooning from this direction may also be helpful.
Myocardial Injury Associated with CTO PCI
It is not uncommon to see small increases in cardiac enzymes associated with retrograde approaches. This occurs due to the ischaemia that is invoked by collateral channel occlusion. Usually, this is well tolerated by the patient and not associated with ischaemia on the ECG. A low-level troponin elevation is of questionable clinical relevance. However, if a dominant collateral channel is used and the patient experiences significant cardiac ischaemia and chest pain when this is crossed, the operator should consider an alternative collateral or strategy to prevent significant myocardial infarction.
During ADR or RDR procedures, it is imperative that attention is paid to large side branches. These should never be sacrificed by the strategy chosen and, where the loss of a significant vessel is possible or likely, an alternative approach should be taken.
When to Stop Revascularisation
It is crucial that the treating physician is capable of recognising futility during the procedure. A contrast (depending on baseline renal function) and radiation limit (many would advocate 4 Gy) should be set and, if no meaningful progress has been made, the procedure should be discontinued. Where a CTO PCI attempt had been made with some progress, dilating the proximal cap and modifying the CTO segment by balloon dilation (an “investment procedure”) can alter the anatomy and facilitate another procedure after a few weeks. Under these circumstances, repeat attempts are associated with very high success rates (~90 %).3
Conclusions
The tremendous improvement of equipment in coronary intervention allows practitioners to revascularise a broad range of CTO lesions in contemporary practice. Many of the most complex lesions will require the use of advanced techniques. CTO interventions can be performed safely in centres willing to make the commitment to training and education to achieve a high level of success for these challenging procedures. A significant part of modern CTO PCI includes an awareness of the potential pitfalls of the procedure, being able toto recognise these events promptly when they arise and the ability to resolve them when they do occur. It is important that operators are suitably trained and centres are appropriately equipped for this.