Valvular Disease
Aortic valve
Severe symptomatic aortic stenosis (AS) bears a dismal prognosis. The mean survival is 2.0 to 4.7 years after the onset of angina, 0.8 to 3.8 years after the onset of syncope and 0.5 to 2.8 years after the onset of congestive heart failure.1 Surgical aortic valve replacement (SAVR) is the mainstay of treatment for these patients.2 In the last decade transcatheter aortic valve replacement (TAVR) has become an accepted alternative in patients for whom surgery would impart a high or prohibitive risk of mortality or morbidity. TAVR does not require openheart surgery. Instead, bovine or porcine pericardial prosthetic leaflets are mounted on a metal frame, which is delivered using a catheter via an endovascular or transapical route. The CoreValve US Pivotal Trial showed absolute mortality benefit of TAVR over SAVR of 4.9 % in high-risk patients (P<0.001 for non-inferiority, P=0.04 for superiority) at one year follow up.3 In the Placement of Aortic Transcatheter Valves 1 trial (PARTNER 1 trial), the absolute mortality benefit in TAVR versus SAVR was of 5.4 % at five years (P=0.76).4
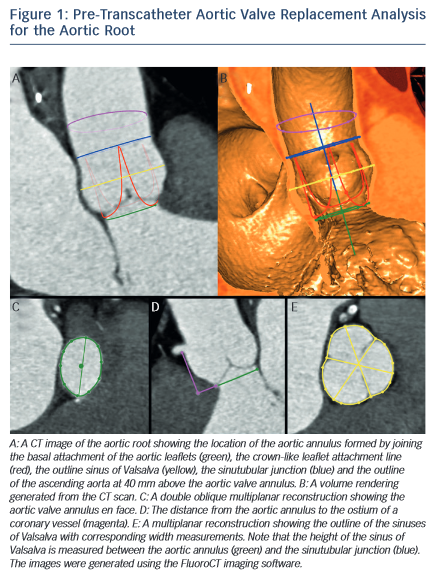
The dimensions of the aortic valve complex are critical for sizing of device (see Table 1 and Figure 1). The aortic annulus, sinuses of Valsalva, ascending aorta, coronary arteries ostia and any bypass grafts should be assessed in all patients considered for TAVR.5 While 2D echocardiography provides valuable haemodynamic information and is readily available, a MSCT-based analysis of the aortic root and vascular access sites has become a sine qua non step in the pre-operative evaluation of patients for TAVR.6 Device sizing using multi-slice computer tomography (MSCT) has been shown to offer more precise measurements of the aortic root than echocardiography and thus reduces rates of paravalvular leak (PVL),7–10 an independent predictor of post-operative mortality from 30 days to 2 years.11–20
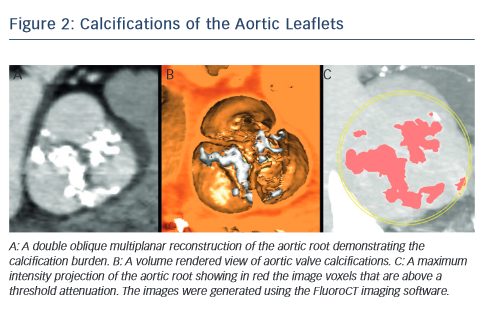
MSCT provides a precise method of evaluating aortic valve calcification (see Figure 2). Calcification is particularly important because it correlates with rates of post-intervention PVL9,21–24 It was recently demonstrated that rates of at least moderate PVL increase with left ventricular outflow tract (LVOT) calcification (OR 2.8, [95 % CI 1.2–7.0], P=0.022), and a leaflet calcium volume greater than 235 mm3 for a threshold of 850 HU (OR 2.8 [95 % CI 1.2–6.7], P=0.023).24
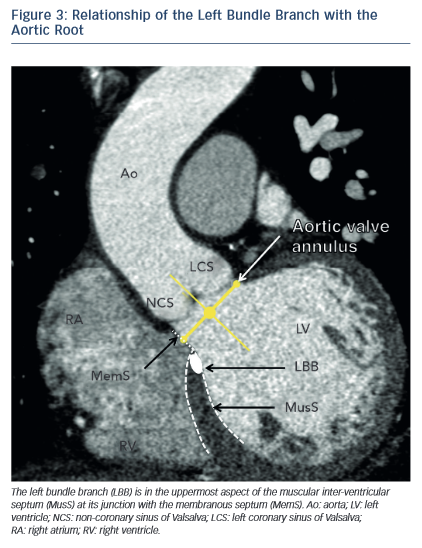
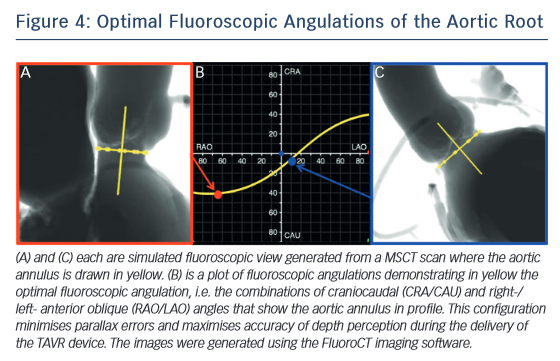
One of the most common complications of TAVR is a new left bundle branch (LBBB) or complete atrioventricular (AV) block requiring the implantation of a permanent pacemaker. The pathophysiology of these iatrogenic conduction abnormalities is the mechanical compression of the left bundle branch in the uppermost aspect of the muscular interventricular septum by the TAVR implant (see Figure 3). An increased depth of implantation is the most frequently identified predictor of LBBB with both balloon- and self-expandable prostheses.25–30 In order to obtain an optimal implantation depth, the operator must minimise parallax inherent to 2D fluoroscopy used during the implantation.31 MSCT can be used to plan optimal C-arm angulations,32–41 i.e. angulations that present the axis of the aortic root parallel to the fluoroscopic detector (see Figure 4). In a recent study,41 such optimised angulations were associated with a significant decrease in implantation time (P=0.0001), radiation exposure (P=0.02), amount of contrast (P=0.001), and risk of acute kidney injury (P=0.03).
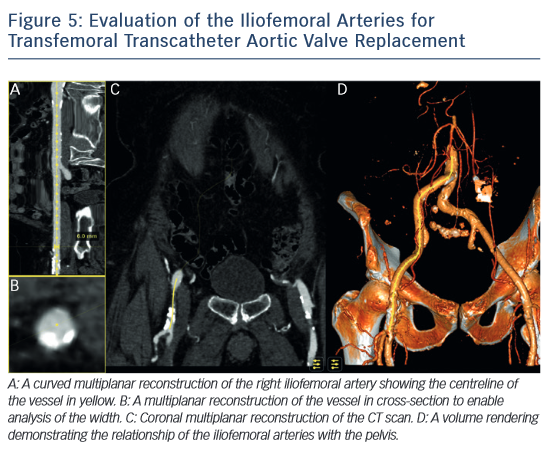
MSCT also plays a role in determining the suitability of access vessels in patients evaluated for TAVR. The majority of TAVR performed today is via the retrograde trans-femoral approach. This technique is the least invasive when compared with trans-apical, trans-aortic, trans-subclavian or trans-carotid TAVR.42-–44 While less invasive, transfemoral TAVR can result in vessel dissection, stenosis, perforation, rupture, arterio-venous fistula, pseudoaneurysm, haematoma or nerve injury.45 The main risk factors of vascular injury secondary to the insertion of the catheter sheath include the vessel diameter, calcification, and tortuosity,46,47 (see Figure 5). Contrast-enhanced MSCT provides information about these three aspects within the same imaging study. The sheath-to-vessel diameter ≥1.05–1.12 is a predictor of vascular injury when measured with CT.47,48 The use MSCT correlates with a decrease in vascular complications.46 MSCT also yields a greater predictive value for vascular complications than plain angiography.48
Mitral valve
Motivated by the success of TAVR, transcatheter mitral valve procedures are seen as the next frontier of structural cardiac interventions for patients at high surgical risk. These procedures consist in the implantation of a device that repairs or replaces the native mitral valve leaflets with the goal of reducing mitral valve regurgitation. The therapies fall into four categories49; edge-to-edge repair (MitraClip), annuloplasty rings (Carillon, Mitralign, Accucinch, Cardioband), chordal implants (NeoChord, V-Chordal) and transcatheter mitral valve replacement (CardiAQ mitral valve, Fortis mitral valve, TIARA mitral valve, Tendyne mitral valve, HighLife mitral valve). These therapies are at various stages of development, from pre-clinical research to commercial availability.
MSCT will likely play a crucial goal in the assessment of the mitral valvular complex with these new therapies. MSCT has been investigated to assess the function and anatomy of the mitral valve. In the context of mitral regurgitation, MSCT can be used to determine the disease etiology,50,51 to quantify the severity,52,53 to describe changes in the geometry of the valvular complex54,55 and to diagnose mitral valve prolapse.56–58
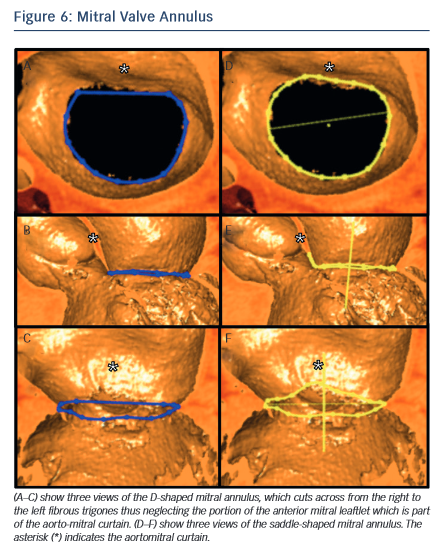
In the context of transcatheter mitral valve replacement (TMVR), MSCT has been proposed to quantify the mitral valve annulus. The mitral valve annulus is often described as either saddle-shaped or as D-shaped (see Figure 6). It has been argued that for some of the devices, in particular those that are not axially symmetrical, the D-shaped annulus may be more appropriate for sizing purposes.59,60 This question remains to be studied, but in the interim we suggest that both techniques be employed.
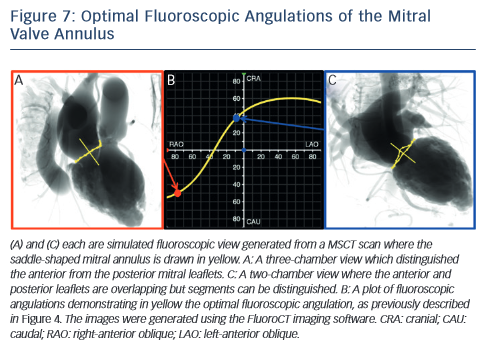
MSCT also plays a role in the optimisation of fluoroscopic viewing angles for mitral valve therapies.31,61 In particular, MSCT allows one to determine the optimal projection curve of the mitral valve annulus (see Figure 7). One may also preselect a fluoroscopic viewing angle corresponding to two-chamber view, which distinguishes mitral valve segments A1P1 from A2P2 and from A3P3 but overlaps the anterior and posterior leaflets such that A1 overlaps P1, A2 overlaps P2, and A3 overlaps P3. Conversely, one may preselect a fluoroscopic viewing angle corresponding to a three-chamber view that distinguishes the anterior from the posterior leaflets but overlaps segments A1,2,3 and separately P1,2,3.
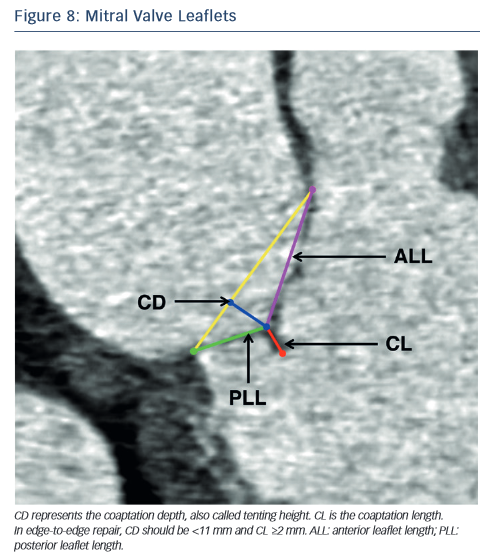
In the context of percutaneous edge-to-edge repair such as the MitraClip procedure, MSCT has been proposed for patient selection. Anatomical criteria essential for this procedure include:62,63 (1) central regurgitant jet located in the A2 and P2 mitral leaflets; (2) in functional mitral regurgitation, a coaptation length ≥2 mm and coaptation depth <11 mm (see Figure 8) and (3) in degenerative mitral regurgitation, a flail gap <10 mm and flail width <15 mm.
As new mitral valve devices undergo clinical trials, MSCT will likely play a critical role in determining patient eligibility. Further research is necessary to determine if MSCT has an impact on procedural success and clinical outcomes of these interventions.
Atrial Septal Defect and Patent Foramen Ovale
The prevalence of a patent foramen ovale (PFO) reaches 25 to 30 % of the general population. In patients having suffered a cryptogenic stroke, the incidence reaches 45.9 %,64 which suggests that PFO may play a significant role in the pathogenesis of cryptogenic stroke.65 Atrial septal defects (ASD) are the most common congenital heart defect found in adult patients.
The indications for defect closure include right ventricular volume overload with a pulmonary-to-systemic flow ratio >2:1, paradoxical embolisation and platypnea-orthodeoxia syndrome. The transcatheter treatment of PFOs and ASDs was first performed in 1975.66 Subsequent studies demonstrated that percutaneous closure has a similar rate of success when compared to surgical closure but offered reduced rates of complications and duration of hospital stay.67–69
Contrast-enhanced MSCT using a saline-chaser can be used to detect and differentiate inter-atrial shunts.70,71 and can also be used to assess disease severity.72 Ostium secundum ASDs, which represent 70 % of cases, are the only type amenable to percutaneous closure. In this context, MSCT can be useful to establish the diagnosis of sinusvenosus type ASD with partial anomalous pulmonary venous return, a condition better treated surgically.73,74
MSCT is also comparable to but less invasive than trans-esophageal echocardiography in the assessment of ASD prior to percutaneous septal occluder implantation.75
Thromboembolic Prophylaxis by Left Atrial Appendage Closure
Atrial fibrillation (AF) is the most common arrhythmia in the general population76 and is an important factor in the pathophysiology of atrial thrombus formation. Chronic anticoagulation is generally indicated in patients with AF and deemed at risk for stroke. A significant number of patients for whom thromboprophylaxis would be indicated are not eligible due to a high bleeding risk. Interestingly, studies suggest that 90 % of thrombus causing strokes in patients with AF originate from the left atrial appendage (LAA).77 This is thought to result from the presence of pectinate muscles within the LAA thus creating an appropriate milieu for blood stasis and thrombus formation. This prompted the development of surgical LAA ligation and eventually, of minimally invasive procedures.
Percutaneous transcatheter LAA closure was first described in 2002 and has since been studied in clinical trials. The Watchman Left Atrial Appendage System for Embolic Protection in Patients With Atrial Fibrillation trial (PROTECT AF trial) demonstrated that the procedure is non-inferior to warfarin therapy but resulted in higher rates of complications such as pericardial effusion.78 Four devices are currently available or under investigation: the WATCHMAN device, the Amplatzer Cardiac Plug, the WaveCrest device and the Lariat epicardial suturesnare delivery device.
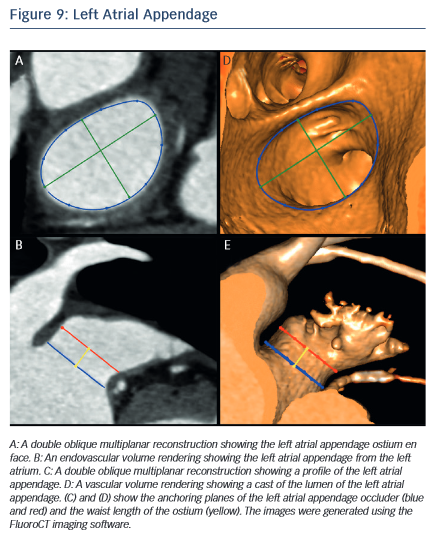
MSCT allows the anatomy of the LAA to be evaluated, which is valuable for device selection,79 assessment of procedural success and longer-term outcomes.80 It has been suggested that the perimeter-derived diameter of the LAA ostium is the most appropriate for device sizing.79 Other important parameters include the minimum and maximum diameters, as well as the waist length of the LAA ostium81 (see Figure 9). MSCT can also be used to determine optimal fluoroscopic angulation for the deployment of the LAA closure device.31
The information provided by MSCT regarding outcomes is complementary to that obtained from transoesophageal echocardiography (TEE). MSCT has a higher rate of detection than TEE for device leaks, which are defined as flow of blood within the LAA past the device.82 The localisation of leaks is also easier with MSCT than with TEE.82 Contrast-enhanced MSCT also enables the evaluation of device thrombus,83 embolisation and pericardial effusion.84,85 Further studies are necessary to provide evidence regarding the impact of MSCT imaging on outcomes of LAA closure.
Conclusions
Herein, we described the utility of MSCT in the context of structural heart interventions. With the fast development of these therapies, MSCT will likely play a role in the development of future sizing algorithms. In the future, interventional cardiologists will likely need to become imaging experts to offer their patients the most optimal outcomes from structural heart interventions.