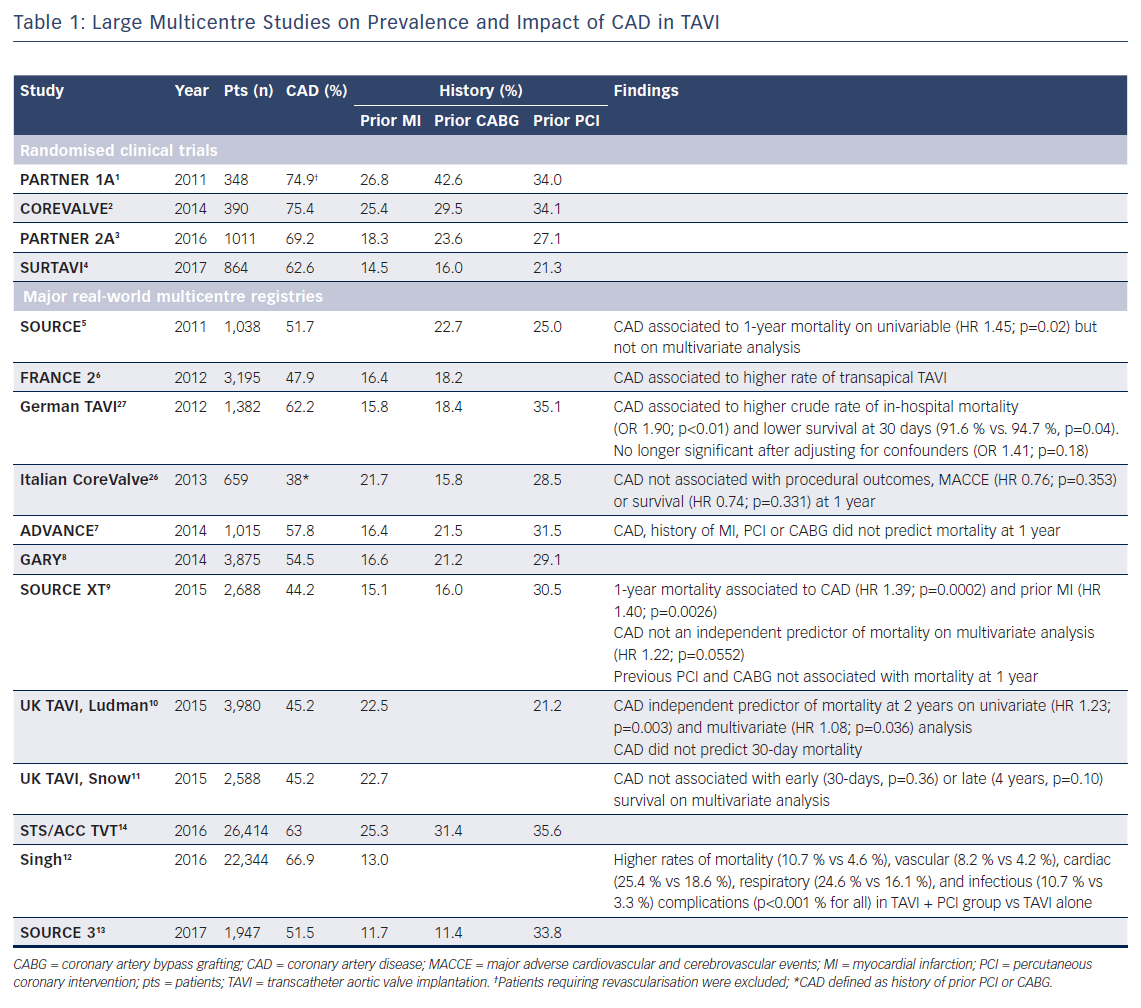
The prevalence of concomitant coronary artery disease (CAD) in patients with severe aortic stenosis (AS) varies widely among different reports. This depends primarily on the definition used to assess CAD as well as study design and patient selection, therefore making a uniform estimate difficult. Major trials investigating transcatheter aortic valve implantation (TAVI) highlighted a substantial majority of high- and intermediate-risk patients (63–75 %) with concomitant CAD.1–4 Contemporary multicentre TAVI registries of real-world patients reported a slightly lower but still considerable incidence of CAD (Table 1).5–13 The large Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) registry revealed that only 37 % of patients undergoing TAVI in the USA are free from significant CAD.14 However, it should be noted that CAD prevalence is often reported according to either angiographic evidence of epicardial coronary stenosis, or based on history of previous myocardial infarction or revascularisation, thereby entailing a risk of overestimation in those previously fully revascularised.
Elderly patients with severe AS complaining of angina frequently exhibit concomitant CAD.15,16 Whether myocardial ischaemia is determined by insufficient oxygen supply due to the underlying valvular disease or due to the presence of CAD is a matter of debate.17 Interestingly, recent studies showed that coronary blood flow and vasodilator reserve are immediately improved after TAVI, with consequent symptom relief.18–21 Of note, due to the mutual worsening of myocardial ischaemia by concomitant CAD and severe AS, clinical manifestations of the two conditions may overlap, rendering the ischaemic relevance of each condition difficult to interpret. Therefore, combined percutaneous coronary intervention (PCI) and TAVI require careful evaluation of the prognostic impact of both CAD and AS.
According to most recent European guidelines, PCI should be considered in patients with significant stenosis of major epicardial vessels undergoing TAVI.22 The objective of the present review is to provide an overview of available evidence on the role of PCI in TAVI patients, focusing on rationale for treatment, patient selection and optimal timing of intervention.
Why Treat: Impact of Coronary Artery Disease on Clinical Outcomes after TAVI
Initial studies exploring the influence of concomitant CAD in patients with severe AS undergoing TAVI reported conflicting results.23–26 This lack of consensus was likely to be a result of several issues such as limited experience with first-generation bioprosthesis, heterogeneity of the study population, small sample size and the absence of a standardised definition of CAD. More recently, different contemporary large-scale registries investigating outcomes in a broad TAVI population found the presence of concomitant CAD to be related to adverse clinical outcomes and impaired survival (Table 1). In an analysis from the German TAVI registry, patients with CAD presented higher crude rate of in-hospital mortality and lower unadjusted 30-day survival compared with those without CAD. However, this difference was no longer significant after adjusting for confounders, suggesting that outcomes could be largely explained by comorbidities.27 Similar findings were reported within the SOURCE XT registry. Although not quite statistically significance on multivariate analysis (HR 1.22; 95 % CI [1.00–1.49], p=0.0552), the presence of CAD at baseline was associated with a higher risk of 1-year mortality.9 Along this line, data from the Bern University Hospital PCI and TAVI registries were extrapolated to analyse three age- and gender-matched cohorts of 248 subjects each. A significantly increased rate of major adverse cardiovascular and cerebrovascular events (MACCE) at 1 year was observed in patients undergoing TAVI with concomitant CAD (16.8 %) compared with TAVI without CAD (9.8 %), and stable CAD undergoing PCI without AS (9.5 %), primarily due to a higher risk of cardiovascular death. Moreover, the first cohort presented more frequently with diabetes mellitus, peripheral artery disease, chronic renal failure and a greater extent and complexity of coronary lesions compared with patients with stable CAD without AS.28
Conversely, results from the UK TAVI registry outlined that CAD, albeit associated with greater comorbid conditions, predicted neither short- nor long-term survival.11 Similarly, in the ADVANCE study, neither CAD, nor history of MI or prior revascularisation were found to be predictors of mortality at 12 months.7
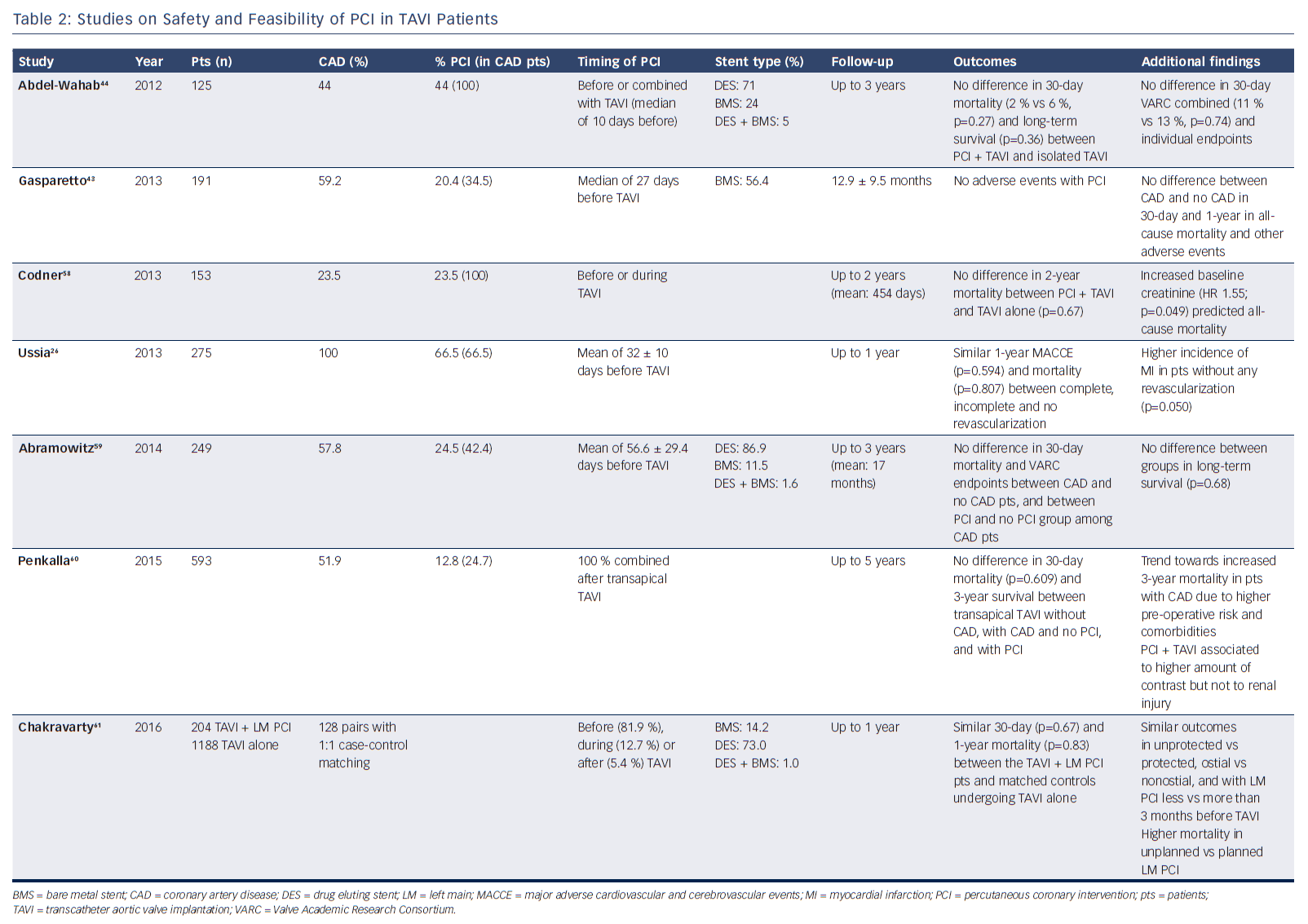
Several small studies showed the feasibility of PCI in TAVI patients (Table 2). A recent meta-analysis by Kotronias and colleagues included 3,858 patients who did or did not undergo PCI prior to or concomitant with TAVI.29 Interestingly, coronary revascularisation was associated with an increased risk of major vascular complications and higher 30-day mortality. However, this association was no longer present at 1 year, suggesting that early adverse outcomes could be ascribed to a worse preoperative risk profile of the PCI group. Indeed, PCI negative impact vanished when outcomes were compared only among studies with 100 % prevalence of CAD in both revascularised and non-revascularised groups. Furthermore, no difference between the two cohorts was observed with respect to 30-day cardiovascular mortality, myocardial infarction and acute kidney injury.
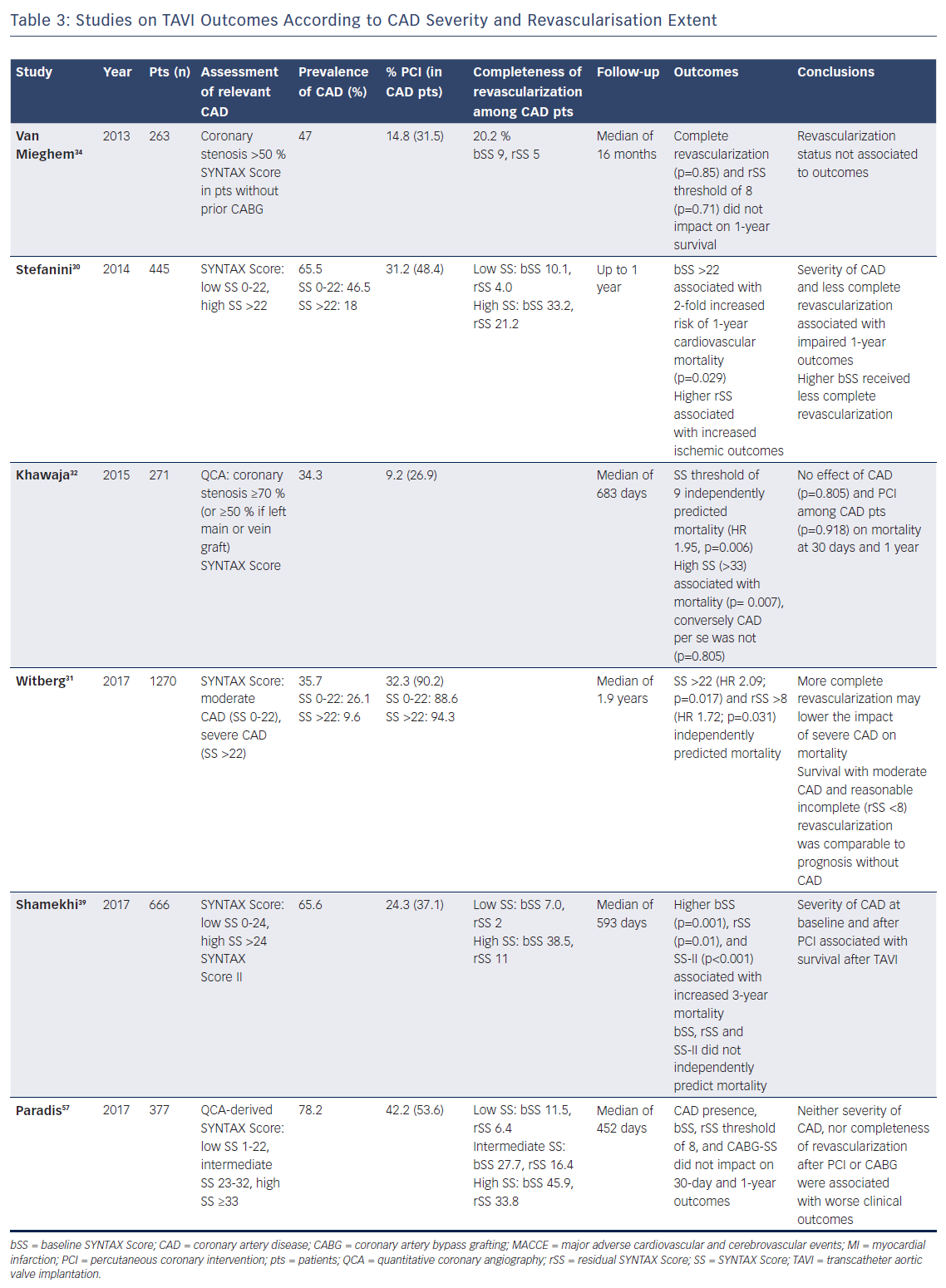
Of note, CAD is a complex and heterogeneous disease, not only due to its frequent association with various comorbidities, but also in terms of extent of myocardium at risk, which may explain the discrepancy between different reports. For this purpose, stratifying patients according to disease severity – i.e. by means of Syntax score – may allow assessment of the prognostic implications of CAD on clinical outcomes after TAVI with greater accuracy (Table 3). In a retrospective analysis of 445 patients from the Bern TAVI registry, severity of CAD was associated with impaired clinical outcomes at 1 year after TAVI. Specifically, this study showed that patients with a Syntax score >22 received less complete revascularisation and had a higher risk of cardiovascular death, stroke or MI than patients without CAD or with a lower Syntax score.30 These findings were confirmed by a recent multicentre study of 1,270 TAVI patients that identified the same threshold of Syntax score >22 as an independent predictor of all-cause mortality (HR 2.09; p=0.017).31 In another observational study, Khawaja and colleagues found a residual Syntax score of 9 to be independently associated with mortality (HR 1.95; p=0.006).32 Consistently, a meta-analysis of 979 patients showed that a low extent of CAD, estimated as baseline or residual Syntax Score ≤10, did not negatively affect outcomes at 30 days and 1 year.33 Moreover, Van Mieghem and colleagues observed no impact on TAVI outcomes according to revascularisation status (complete or incomplete), nor by a residual Syntax score threshold of 8.34 Based on these observations, the authors concluded that only a carefully selective PCI of major proximal epicardial vessels subtending large areas of myocardium at risk is likely to confer a clinical benefit.
Taken together, these findings suggest that severe CAD, often encountered in association with a wide range of comorbid conditions, may negatively affect survival of patients with AS undergoing TAVI. Therefore, reducing myocardial ischaemic burden through PCI appears a legitimate option to improve clinical outcomes after TAVI.
Who to Treat: Assessment of Relevant Coronary Artery Disease
Non-invasive Assessment
Assessment of myocardial ischaemia due to concomitant CAD in the presence of severe AS can be challenging because of the overt difficulties in interpreting symptoms when both diseases coexist. In patients with suspected CAD, exercise or pharmacological stress examination is essential in determining inducible ischaemia, which may benefit from coronary revascularisation. However, these tests are usually contraindicated in patients with severe AS. Nonetheless, dobutamine echocardiography is accepted in cases of low-gradient AS to assess disease severity and guide treatment. Moreover, stress echocardiography is a prognostic tool for risk stratification by appraising contractile reserve in patients with severe AS and impaired left ventricular function.
Studies investigating safety and diagnostic accuracy of non-invasive testing with vasodilator stressor in severe AS demonstrated an overall feasibility due to absence of major adverse cardiac events related to the test, although specificity of results might be lower than in the absence of AS.35 In fact, dipyridamole echocardiography on patients with severe AS and normal coronary artery may elicit an ischaemic susceptibility arising only from the aortic valve disease, which is possibly reversible after treatment of AS.36
Coronary Angiography
Angiographic screening of coronary anatomy is recommended prior to any aortic valve intervention. Indication and survival benefit attributed to coronary artery bypass grafting (CABG) of ≥50 % coronary stenosis in subjects who are candidates for surgical aortic valve replacement has been established for decades.37,38
Given the importance of assessing CAD severity to properly evaluate its clinical significance, coronary angiography allows quantification of the disease extent and complexity. For this purpose, the Syntax score represents an attractive tool for decision making also in patients with AS. Available evidence indicates that CAD in patients without a high ischaemic burden may bear a neutral effect on outcomes after TAVI. Indeed, as summarised above, several studies showed that survival of patients with moderate CAD and reasonably incomplete revascularisation was comparable with the prognosis of patients without CAD (Table 3).30,31,39
Owing to its high negative predictive value in the detection of CAD, computed tomography angiography, regularly performed preoperatively to evaluate vascular access, annular and aortic root measurements, might also be useful for CAD assessment in TAVI patients. Indeed, Chieffo and colleagues reported that only 22 % of patients required additional coronary angiography after first-line CAD screening with coronary computed tomography angiography in a single centre registry.40
Invasive Functional Assessment
Functional assessment of coronary lesions by means of fractional flow reserve (FFR) has been shown to accurately guide coronary revascularisation, determining clinical, rather than anatomical, relevance of flow-limiting stenosis. FFR evaluation is based on pressure-flow measurements during adenosine-induced maximal hyperaemia. As well as safety concerns related to vasodilator administration in AS, diagnostic accuracy of FFR has been arguable due to potential underestimation of functionally relevant intermediate stenosis secondary to haemodynamic changes derived from aortic valve disease.35 Although apparently clinically feasible, a series of minor coronary flow variations were observed with FFR after TAVI, suggesting that borderline coronary lesions could become significant followingthe procedure.41
Alternatively, avoiding induced hyperaemia through instantaneous wave-free ratio (iFR) may be advantageous, increasing safety and reliability of functional evaluation of critical CAD in TAVI patients. Some authors also advocated a hybrid approach, opting for adenosine-independent iFR as screening tool, while sparing the assessment of borderline lesions by means of FFR only after TAVI.42
When to Treat: Timing of Percutaneous Coronary Intervention
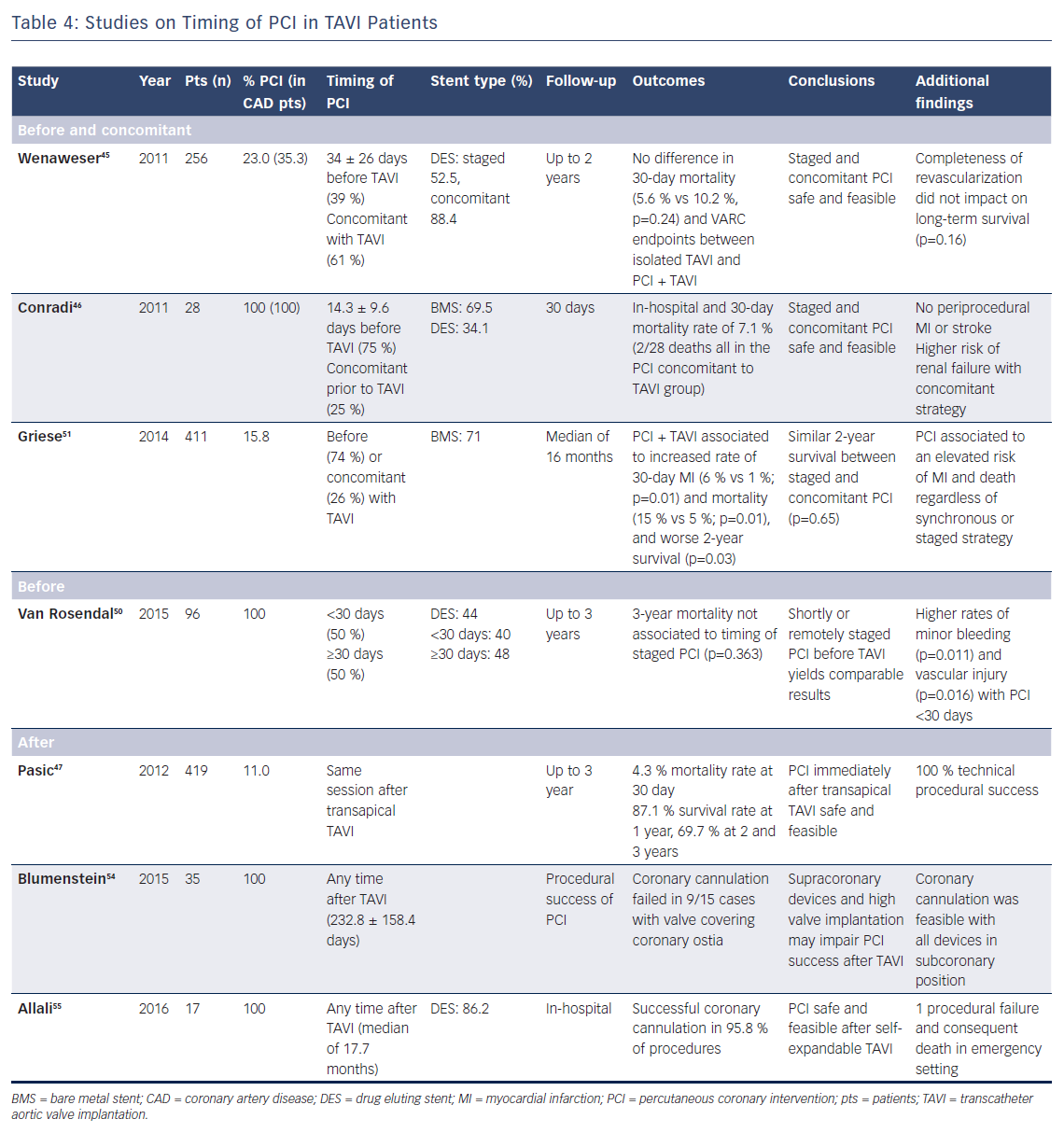
Available data seem to suggest that PCI both before and along with TAVI are feasible and do not impact on early survival, but there are a few caveats.43–47 However, there is very limited evidence in support of staging PCI after TAVI (Table 4).
Before Transcatheter Aortic Valve Implantation
PCI can be safely performed in patients with severe AS, although low ejection fraction and high STS score determine a higher risk of short-term mortality.48
Patients with stable CAD at high bleeding risk undergoing coronary stent implantation require dual antiplatelet therapy for a minimum of 3 months, yet a 1 month duration may also be considered.49 Performing early staged TAVI after PCI may result in an additional periprocedural haemorrhagic risk due to the ongoing antithrombotic therapy. Conversely, postponing TAVI until antithrombotic therapy cessation raises concerns about the delay of definitive treatment for severe symptomatic AS. Moreover, a delayed approach would further prolong patient exposure to dual antiplatelet therapy for 3–6 months immediately after TAVI, as suggested by current guidelines.22
A study investigating optimal timing of PCI prior to TAVI stratified 96 patients in one group undergoing PCI ≥30 days before TAVI and another group undergoing PCI within 30 days before TAVI. Interestingly, the shortly staged PCI (<30 days) cohort was at higher risk of minor bleeding events and vascular injury after TAVI, although the lower haemoglobin level and higher incidence of atrial fibrillation at baseline in this group might have contributed to these findings.50 Along the same lines, it appears reasonable to postpone TAVI until vascular access healing has occurred and enough time has passed since antiplatelet loading dose administration.
Finally, hypotension from rapid ventricular pacing has often been considered to potentially worsen myocardial ischaemia, therefore revascularisation before TAVI would allow mitigation of a patient’s procedural risk.
Concomitant with Transcatheter Aortic Valve Implantation
Beyond reducing costs of intervention and rehospitalisation, another potential advantage of single-staged PCI and TAVI is the use of the same transartierial access for both procedures, with a lower consequent risk of vascular and bleeding complications. Although higher rates of periprocedural mortality, vascular complications and myocardial infarction have been reported in patients undergoing combined TAVI and PCI, no significant difference between staged and concomitant procedures was observed.29,51 However, a trend towards a reduced incidence of bleeding and vascular complications was noted in patients undergoing PCI and TAVI in the same session.45,51 Nevertheless, it must be acknowledged that studies are heterogeneous and no uniform information regarding ongoing therapy and time interval between the two procedures was provided.29
There is also legitimate apprehension of concomitant PCI because of nephrotoxicity due to a prolonged intervention. Indeed, patients undergoing simultaneous PCI and TAVI may receive a higher amount of contrast agent, therefore being more exposed to renal injury.46,52 However, correlation between contrast volume and acute kidney injury is weak in TAVI patients and many studies failed to show its predictive value.53
After Transcatheter Aortic Valve Implantation
Since TAVI has been shown to improve coronary perfusion and anginal symptom relief, evaluation of CAD after valve intervention may allow a more physiological and accurate assessment of myocardium at risk of ischaemia, especially in the presence of borderline coronary lesions. However, this strategy entails a series of technical challenges. Coronary cannulation and catheter manipulation may be hampered later after valve deployment, especially with self-expandable prosthesis due to the bulgy stented frame placed over coronary ostia. Malpositioning of the valve, mainly in the case of high implantation and consequent aortic root distortion, are essential factors raising this risk.54 More importantly, compromised cannulation of coronaries could be ominous in an emergency situation.55 Notwithstanding, a recent study reported technical success in all 46 cases of PCI performed right after balloon-expandable TAVI within the same operative session, without additional procedural complexity.47 This suggests that in the presence of extensive CAD and the possibility of future need for PCI, valve selection – i.e., favouring bioprosthesis with subcoronary implantation – may be crucial to allow coronary accessibility after TAVI.
Conclusion
CAD is frequent among TAVI patients and PCI is commonly performed in this setting. However, assessment of relevant CAD in the presence of severe AS can be difficult and symptoms potentially misleading. Available evidence in support of different revascularisation strategies is mostly based on retrospective, single-centre studies reporting unadjusted and discordant outcomes. The ongoing percutaneous coronary intervention prior to transcatheter aortic valve implantation (ACTIVATION) study is the first randomised controlled trial designed to evaluate non-inferiority of PCI compared with not treating such coronary lesions before TAVI.56
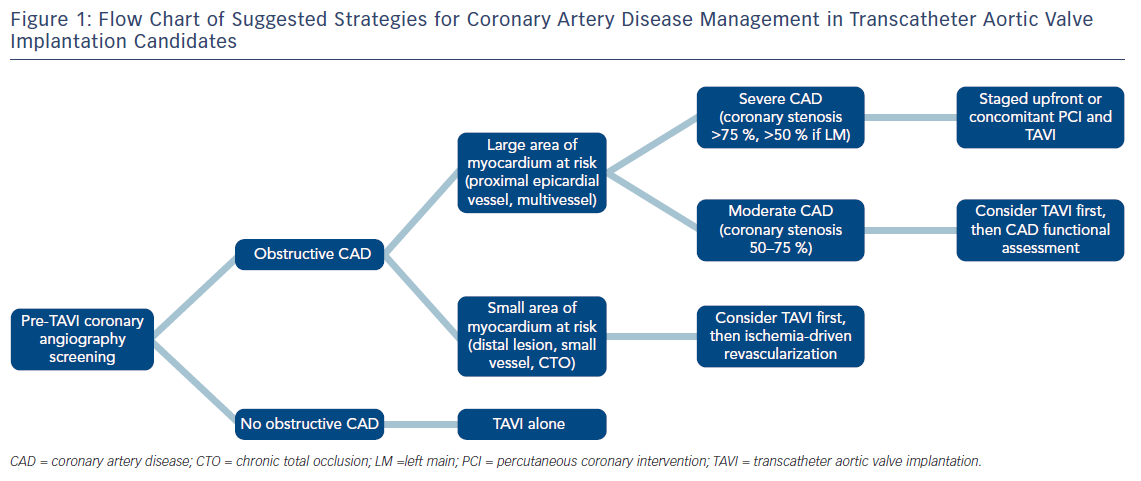
While awaiting further data to provide solid recommendations, the decision to pursue coronary revascularisation in TAVI patients should be tailored case-by-case and based on clinical and anatomical variables, appraised by multi-disciplinary consensus within the local Heart Team. For this purpose, Syntax score may help stratify CAD severity to guide selection of patients who will benefit the most from concomitant PCI. At this point, it is reasonable to foster selective revascularisation strategies of proximal epicardial coronary arteries in subjects with signs of high ischaemic burden (Figure 1). Finally, staging PCI either before or concomitant with TAVI appears feasible. Optimal timing should be decided on an individual basis, taking into consideration patients and procedural characteristics.
Overall, current management of CAD in TAVI patients is largely based on observational evidence. Given the broad expansion of TAVI indication in lower risk patients, large and well-designed randomised trials investigating strategies for the optimal management of CAD in TAVI patients are needed.