Coronary artery disease (CAD) remains a major cause of morbidity and mortality despite major improvements in primary and secondary prevention strategies. Percutaneous coronary intervention (PCI) or surgical revascularisation may be indicated in many patients with acute or stable CAD.1
Since the first coronary intervention using catheter mounted balloons, percutaneous treatment of CAD has evolved from percutaneous balloon angioplasty, PCI using bare-metal stents (BMS) or first-generation drug-eluting stents (DES) to second and newer-generation DES implantation.2–5 The improvements in newer-generation DES have resulted in better safety and efficacy compared with older DES and BMS. However, the efficacy of stents is restricted in small vessel disease (SVD).6–8
SVD is common among patients undergoing PCI and has been documented in up to 30% of cases.9–11 Myocardial revascularisation of small vessels remains challenging owing to increased rates of technical failure following coronary artery bypass graft surgery and an increased risk of restenosis with PCI resulting in repeated interventions.12,13 SVD also remains an independent predictor of major adverse cardiac events (MACE).14
Drug-coated balloons (DCBs) are a novel concept for the treatment of CAD and an established therapeutic option for in-stent restenosis (ISR) of BMS and DES (Figure 1).15,16 The technique is based on the fast delivery of highly lipophilic drugs to the vessel wall after single balloon inflation.6 The efficacy and safety of DCBs in native SVD has recently been demonstrated in a large study with clinical endpoints, which showed a similar rate of MACE after 12 months in patients treated either with a DCB or second-generation DES.17
Methods
This review includes all English language studies after a detailed search of PubMed according to established methods and in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews and meta-analyses in healthcare interventions.18 The following keywords were used: “small” AND “coronary” AND “drug-eluting stents” OR “DES” OR “DCB” OR “DEB” OR “drug-eluting balloons” OR “drug-coated balloons”. Databases were screened up until 4 February 2019. The most up to date and inclusive data for each study were used for abstraction. References of original and review articles were cross-checked.
Definition and Prevalence of Small Vessel Coronary Artery Disease
Several definitions of SVD have been proposed. However, most recent studies have identified an angiographic reference vessel <3 mm as the most appropriate cut-off.19 Therefore, it can be stated that any coronary vessel amenable to percutaneous treatment with a 2.75 mm or smaller device should be considered ‘small’.17 Some authors have suggested the term ‘very small vessel coronary artery disease’ for those coronary vessels that are amenable to percutaneous treatment with a 2 mm device.20
Despite varying definitions in the literature, there is universal agreement that SVD is common, being prevalent in up to 30% of patients with symptomatic CAD, and that patients with diabetes or chronic renal failure are at even higher risk of developing this type of CAD.21,22 The prognostic implications of SVD are important since a small reference vessel diameter in the coronary segment undergoing PCI is significantly and directly associated with an increased risk of adverse clinical events, including ISR and stent thrombosis (ST) compared with larger reference vessel diameters.9,23
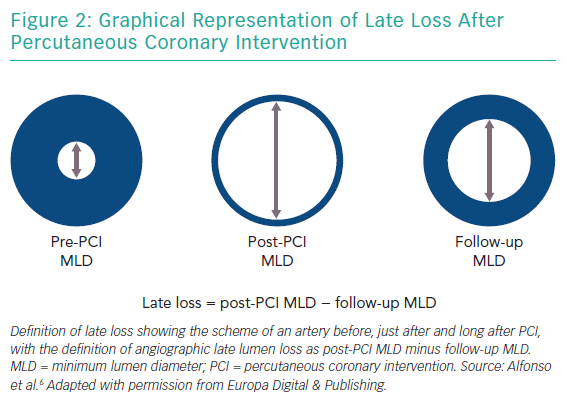
The goal of PCI is to improve the minimum lumen diameter in a given target coronary segment, which has a specific reference vessel diameter (roughly defined as the average of the diameters of apparently normal segments localised proximally and distally to the target segment).20 Thus, the minimum lumen diameter increases significantly after the procedure but decreases at follow-up, mainly because of recoil and hyperplasia phenomena (Figure 2).
Stent implantation results in arterial injury, initiating a vasculo-proliferative cascade with smooth muscle cell proliferation and migration resulting in neointimal hyperplasia. The amount of neointimal hyperplasia is largely independent of vessel size, and thus the absolute amount of late lumen loss – an angiographic measure of neointimal hyperplasia – is similar across a wide range of vessel diameters.24 Therefore, small vessels are more prone to restenosis than larger vessels because they are less able to accommodate neointimal tissue without compromising blood flow.25
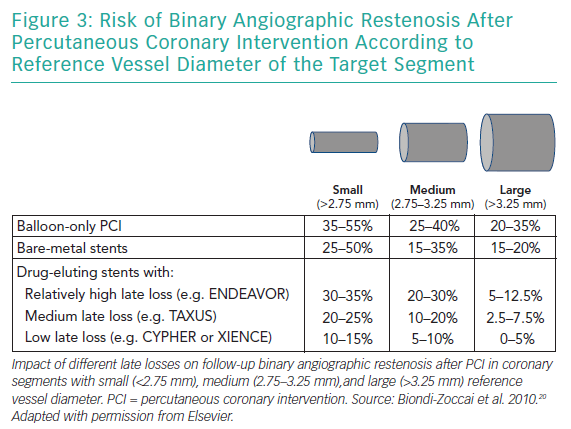
Late lumen loss is defined as the difference between the postprocedural minimum lumen diameter and minimum lumen diameter at follow-up, and ranges between 0.05 and 0.10 mm (for the most effective DES) and 1.0 and 1.5 mm (for balloon-only PCI; Figure 2).20,25 While a low late loss is generally beneficial, it appears even more important in SVD (Figure 3).
Drug-coated Balloons: The Technology
DCBs have been developed to overcome some of the limitations of DES, especially for patients with SVD.6,7,8,26 DCBs are semi-compliant angioplasty balloons covered with an antiproliferative drug that is rapidly released from a lipophilic matrix upon contact with the vessel wall. Mechanical expansion of the vessel is combined with release of an antiproliferative drug without leaving a foreign body. The development of DCBs is complex and factors other than the active drug itself contribute to its effect. The lipophilic matrix must maintain the drug on the balloon during transit to the lesion, while at the site of dilatation it should ensure a rapid and homogenous drug transfer to the vessel wall.
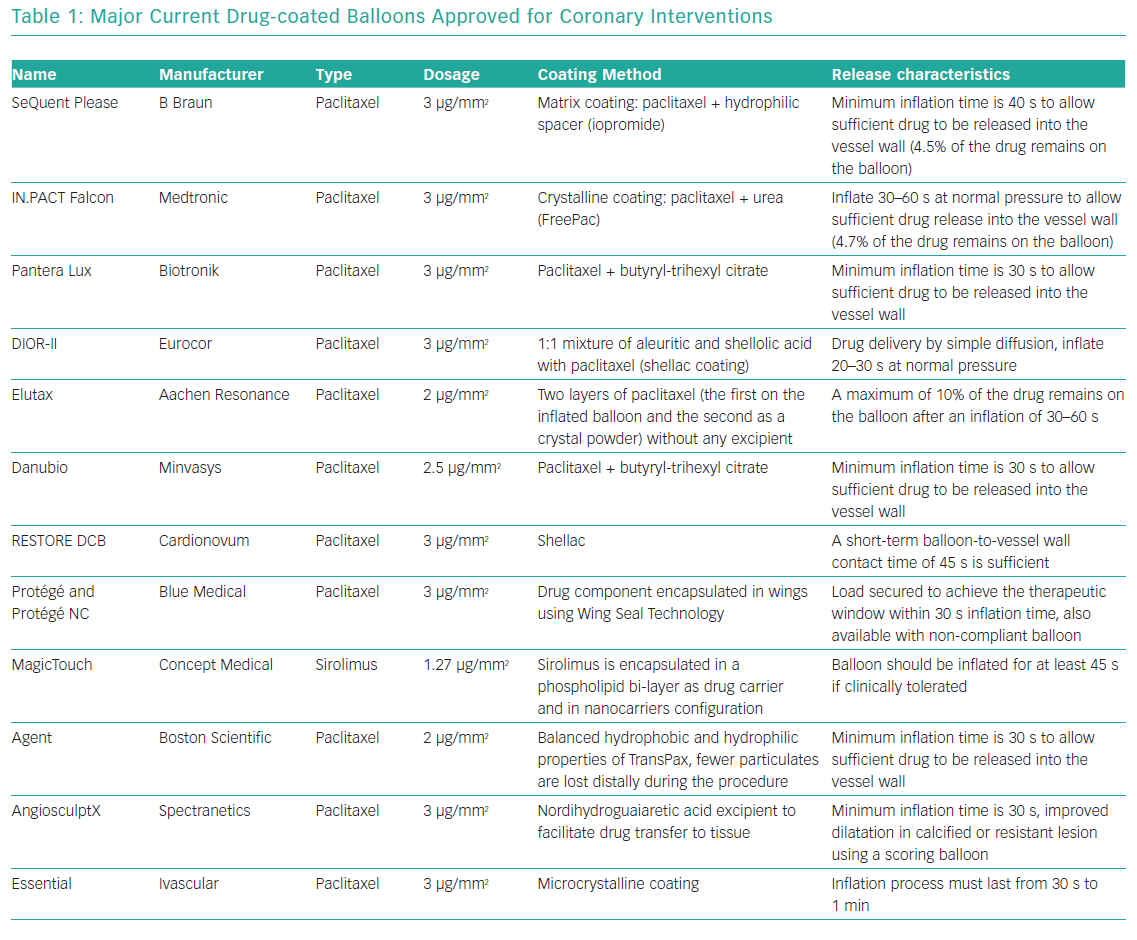
Pharmacokinetic characteristics of each DCB largely depend on the carrier and manufacturing process used to develop the coating. A variety of carrier excipients, such as iopromide, urea and shellac, among others, have been investigated to determine their ability to enhance drug delivery.27 Most currently available DCBs use paclitaxel because of its highly lipophilic profile, potent antiproliferative effect and chemical stability after delivery.28 The -limus drugs (sirolimus and zotarolimus), which are much less lipophilic than paclitaxel, have also shown some efficacy in the suppression of neointimal growth in a limited number of animal models.29,30 Several types of DCBs have been introduced in the market and approved for coronary use (Table 1). Current research is focused on different coatings and drug delivery technologies using -limus drugs and nanocarriers to identify optimal nanoparticle structure for efficient transfer of drugs to all layers of the vessel wall, achieving high tissue concentrations that persist days after application with a low rate of systemic drug leak.30,31 However, clinical data are still sparse.
The DCB technique has been successfully tested in ISR, where good clinical efficacy was demonstrated in most studies, but not in all.15,16,32–39 Current guidelines recommend the use of DCBs for patients with coronary ISR (class I, level of evidence A).1,40 In addition, DCBs are now increasingly being seen as an attractive option for the treatment of SVD and bifurcation lesions.27,28 Native vessels treated by DCBs, due to absent metallic struts and polymer, keep their vasomotion properties reducing abnormal flow patterns without the risk of ISR and late ST, and remain possible targets for coronary artery bypass grafts.41
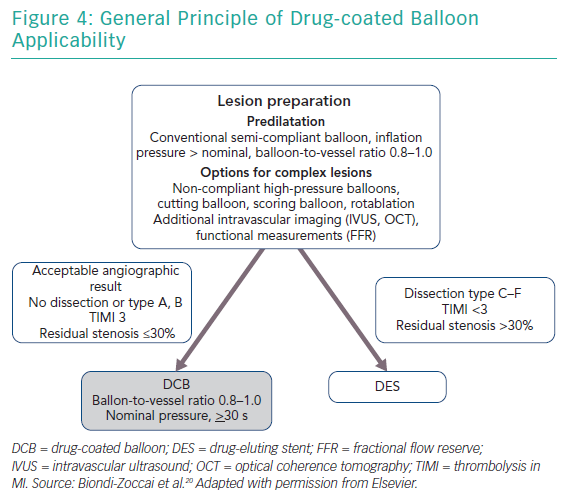
After lesion preparation, taking care to avoid geographic mismatch, a DCB is inflated at nominal pressure for at least 30 seconds. Implantation of a DES is recommended in case of a major dissection (type C or higher), a residual stenosis of >30%, or reduced flow (Figure 4).
Although previous studies have not been powered to determine the optimal duration of dual antiplatelet therapy (DAPT), the available data suggests that it is required for a shorter time after DCBs compared with DES, which may be beneficial in many patient groups. The lack of a metallic scaffold and the shorter period of post-procedure inflammation, virtually eliminates the risk of thrombosis.42 Most studies with DCBs in ISR or SVD used a short period of DAPT of only 1 month, without a higher rate of thrombotic events.15,17,33,43 Thus, a short period of DAPT in patients with stable coronary artery disease treated with DCBs alone seems to be safe and effective.
Clinical Trials of Drug-coated Balloons for Small Vessel Coronary Artery Disease
In a retrospective analysis by Sinaga et al. in 335 patients receiving either DCB (SeQuent Please, B Braun; n=172) or a second-generation DES (n=163), with a device diameter of ≤2.5 mm,44 there were no differences between baseline demographics or concomitant disease between the groups. There was no difference in MACE (12% versus 12%; p=1.00) and target lesion revascularisation (5% versus 4%; p=0.60) after 12 months. Pre-dilatation was done in all patients. Rates of bailout stenting were not reported.
Sim et al. retrospectively analysed 287 patients receiving either the SeQuent Please DCB (n=87) or a second-generation DES (n=200).45 Patients received either a 2 mm DCB or a 2 mm DES. Bailout stenting was necessary in seven patients in the DCB group. At 12 months, target lesion revascularisation (5% versus 6%; p=0.74) and death from any cause (5% versus 9%; p=0.24) were similar between the groups.
Venetsanos et al. analysed a large cohort of 7,655 patients who received either a DCB (n=1,197) – SeQuent Please, IN.PACT Falcon (Medtronic-Invatec) or Pantera Lux (Biotronik) – or a second-/third-generation DES (n=6,458) – Xience (Abbott Laboratories), Promus and Synergy (Boston Scientific), Resolute (Medtronic), Orsiro (Biotronik) or Nobori (Terumo).46 Median follow-up was 901 days. DCB patients were older with a higher cardiovascular risk profile. Bailout stenting after DCB was performed in 8% of lesions. Rate of target lesion revascularisation and target lesion thrombosis was 7.0 versus. 4.9% and 0.2 versus 0.8% for DCB versus DES, respectively. After propensity score matching the adjusted risks of target lesion revascularisation were not significantly elevated (HR 1.05; 95% CI [0.72–1.53]). However, DCB was associated with a significantly lower risk of target lesion thrombosis compared with DES (adjusted HR 0.18; 95% CI [0.04–0.82]).
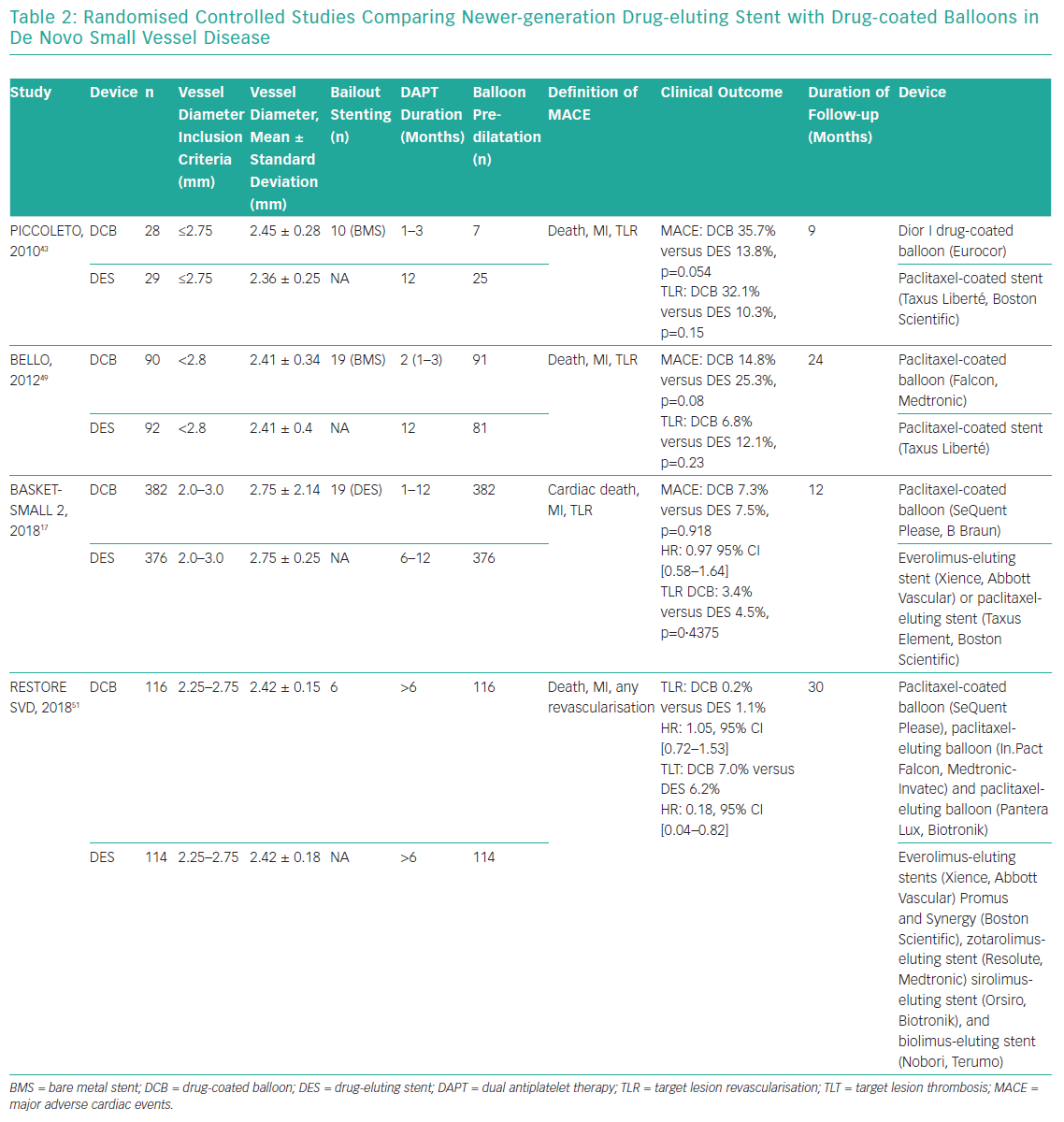
There are few randomised controlled studies comparing newer generation DES with DCBs in de novo SVD (Table 2). The Paclitaxel-Coated Balloon Versus Drug-Eluting Stent During PCI of Small Coronary Vessels (PICCOLETO) study randomised 57 patients with SVD (diameter ≤2.75 mm) in a 1:1 fashion to either a paclitaxel-eluting balloon (n=28; Dior, Eurocor) or to a first-generation paclitaxel-eluting stent (n=29; Taxus, Boston Scientific).43 The study was interrupted after including only 57 patients based on an interim analysis which showed a higher rate of target lesion stenosis after 6 months (DCB 44% versus DES 24%, p=0.029) and higher MACE rates (36% in DCB versus 14% in DES, p=0.054), mainly driven by higher target lesion revascularisation rates in DCB versus DES (32% versus 10%, p=0.15). However, this result was felt to reflect a lack of efficacy of the DCB used, rather than a class effect of DCBs overall.47 In first-generation DCBs, adherence of paclitaxel was mediated by the roughened surface of the balloon, providing a significantly lower drug concentration in the tissue and accordingly lower inhibition of neointimal proliferation. Paclitaxel is released more completely and homogenously after the first balloon expansion from newer generation DCBs, resulting in high bioavailability in the target lesion.48
The Balloon Elution and Late Loss Optimization (BELLO) study randomised 182 patients with lesions in small vessels (<2.8 mm) to either paclitaxel-coated balloon (n=90; IN.PACT Falcon and provisional BMS) or paclitaxel-eluting stent (n=92; Taxus Liberté, Boston Scientific), as per standard practice.49 Bailout stenting was required in 20% of patients in the DCB arm. At 6 months, DCBs and DES were associated with similar rates of angiographic restenosis (10% versus 14.6%; p=0.35), target lesion revascularisation (4.4% versus 7.6%; p=0.37), and MACE (10% versus 16.3%; p=0.21). The clinical efficacy of DCBs was confirmed after up to 3 years, showing a trend toward improved outcomes with regard to MACE.50 BELLO demonstrated the importance of routine predilatation, which was performed in 96.8% of interventions compared with 25% in the PICCOLETO study.
In the Basel Kosten-Effektivitäts trial, Drug-Coated Balloons for Small Coronary Artery Disease (BASKET-SMALL 2), 758 patients with SVD (<3 mm) were randomly allocated to receive treatment either with a paclitaxel-coated balloon (n=382; SeQuent Please) or one of two second-generation DES (n=376), the paclitaxel-eluting Taxus Element stent or the everolimus-eluting Xience Stent (Abbott Vascular).17 Lesion preparation was mandatory, and randomisation was only possible if angiographic criteria were met (no high-grade dissection, no reduced blood flow and residual stenosis ≤30%). The rate of MACE after 12 months did not differ between the two groups (7.3% for DCB versus 7.5% for DES, p=0.92). Furthermore, the individual components of the primary endpoint did not differ between the two groups (DCB versus DES: cardiac death 3.1% versus 1.3%, p=0.11; non-fatal MI 1.6% versus 3.5%, p=0.11; and target vessel revascularisation 3.4% versus 4.5%, p=0.448). Bailout stenting was required in 5% of patients in the DCB arm. The study was only powered for clinical endpoints, not for angiographic endpoints.
The Drug-Coated Balloon Versus Drug-Eluting Stent for Small-Vessel Disease (RESTORE-SVD) study compared 230 patients with SVD (vessel diameter ≥2.25 mm and ≤2.75 mm) with a new generation paclitaxel-coated balloon (n=116; Restore, Cardionovum) versus a zotarolimus-eluting stent (n=114; Medtronic).51 The trial was designed to evaluate non-inferiority of DCB versus DES for a 9-month primary angiographic endpoint, and assessed in-segment percent diameter stenosis, which was similar with 29.6 ± 2.0% versus 24.1 ± 2.0% (p for noninferiority <0.001). There were no differences in target lesion revascularisation, cardiac death, MI and a composite endpoint consisting of all-cause death, MI and any revascularisation. Pre-dilatation was done routinely in all patients. In the DCB group, 5% (6 of 116) needed bailout stenting. The DCB and DES groups had comparable 1-year rates of target lesion failure (4.4% versus 2.6%, p=0.72).
A meta-analysis of 1,824 patients from seven studies (four randomised controlled trials and three observational studies) compared DCBs (n=759) with other modalities of treating de novo SVD (n=860). DCBs were associated with a similar risk of target lesion revascularisation (OR 0.99; 95% CI [0.54–1.84], p=97) and MACE (OR 0.86; 95% CI [0.51–1.45], p=0.57) during a mean follow-up of 7 ± 1.5 months, compared with DES. DCBs were associated with a significantly lower risk of TLR (OR 0.19; 95% CI [0.04–0.88], p=0.03) and binary restenosis (OR 0.17; 95% CI [0.08–0.37], p=<0.00001) compared with non-coated balloon angioplasty.52
Conclusion
The treatment of CAD with DCBs benefits from local drug delivery and a ‘leave nothing behind’ strategy. Many experimental and clinical studies have demonstrated that DCBs are safe and effective for certain indications. While evidence of the value of DCBs in patients presenting with ISR is overwhelming, DCBs also appear promising for selected de novo coronary lesions in SVD. Further studies are required to ascertain the long-term benefits of DCB compared with new-generation DES in this setting before unrestricted use of DCB can be recommended.