Dual antiplatelet therapy (DAPT), defined as the use of a P2Y12 receptor inhibitor (clopidogrel, ticagrelor or prasugrel) and aspirin, is required after percutaneous coronary intervention (PCI) with drug-eluting stents (DES).1 Although the use of DES has been shown to reduce the rate of restenosis as compared with bare-metal stents (BMS), there is concern that DES may be associated with a higher risk of late and very late stent thrombosis (ST),2,3 particularly after DAPT discontinuation.4 DAPT prevents thrombotic complications through a double mechanism. First, DAPT protects the stented segment from ST, which occurs as a result of inflammation during healing.5,6 Second, DAPT confers protection from atherothrombotic events occurring outside the stented segment, lowering the risk of recurrent MI.4,7 The current American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines/Society for Cardiovascular Angiography and Interventions (ACCF/AHA/SCAI) guidelines on PCI use recommend at least 12 months of DAPT after DES implantation.8 The European Society of Cardiology (ESC) guidelines endorse 6–12 months of DAPT after DES implantation,1 and the ESC and the European Association for Cardio-Thoracic Surgery (ESC/EACTS) recommend 12 months for all patients with acute coronary syndrome (ACS) irrespective of revascularisation strategy.9 However, the optimal duration of DAPT post-DES implantation remains poorly defined.
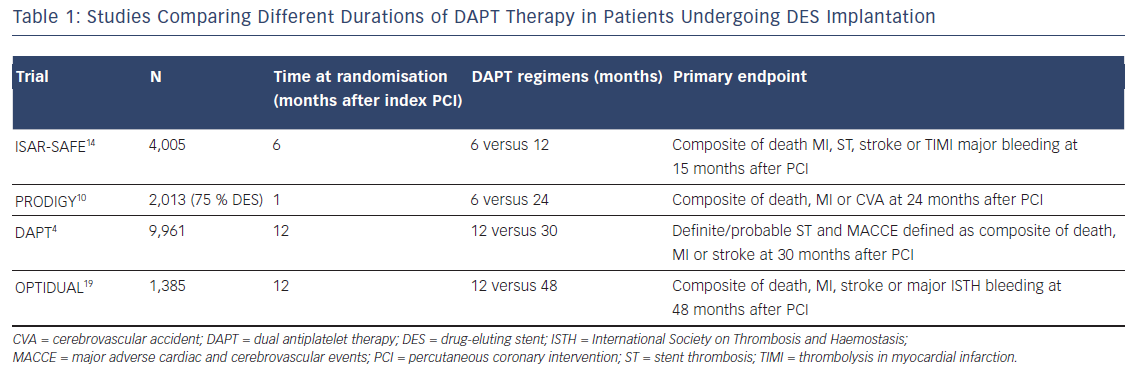
The most recent clinical trials (see Table 1) and meta-analyses in patients who underwent a PCI with stent implantation, have highlighted two key concepts: first, the hazard rate for ischaemic events is not increased with reduced (<12 months) compared with standard (12 months) or prolonged (>12 months) DAPT duration, especially with newer-generation DES10–12 and second, prolonged DAPT reduces the rate of ischaemic events at the cost of increased risk of bleeding.4,13
Studies Evaluating Reduced Duration of Dual Antiplatelet Therapy
In the Prolonging Dual Antiplatelet Treatment After Grading Stent- Induced Intimal Hyperplasia Study (PRODIGY) trial, consisting of a population predominantly presenting with unstable coronary artery disease, the use of DAPT for 24 months in patients who had received DES (75 %) was not significantly more effective than a 6-month clopidogrel regimen followed by aspirin monotherapy in reducing the risk of MI or cardiac death.10 However, 24 months of clopidogrel therapy resulted in a significant increase in the number of bleeding episodes, including life-threatening events. In the Safety And Efficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) trial, 6 months of DAPT were related to similar net clinical outcome compared with 12 months of DAPT after PCI with a DES.14 Due to a slow enrolment and low event rates, the trial was stopped prematurely after enrolment of 4,005 of the 6,000 planned.
The safety of a reduced DAPT duration compared with 12-month DAPT was confirmed in a meta-analysis of 10 randomised clinical trials (n=32,287), where reduced DAPT duration regimen after PCI with DES was associated with a significant reduction in the rate of major bleeding with no significant differences in ischaemic or thrombotic outcomes.15 On the contrary, prolonged DAPT duration reduced the incidence of thrombotic complications, including ST and MI, at the cost of increased rates of major bleeding.
The Zotarolimus-eluting Endeavor Sprint Stent in Uncertain DES Candidates (ZEUS) trial was the first to show that zotarolimus-eluting stent implantation followed by a reduced DAPT duration (median duration of 32 days) resulted in a lower risk of major cardiovascular events, compared with BMS, in a selected population of patients with stable coronary artery disease (SCAD) or ACS at high bleeding or thrombosis risk or at low risk of restenosis.16 Lastly, the recent Prospective Randomized Comparison of the BioFreedom Biolimus A9 Drug-Coated Stent versus the Gazelle Bare-Metal Stent in Patients at High Bleeding Risk (LEADERS FREE) trial, involving patients at high bleeding risk who underwent PCI and were treated with a reduced (1-month) DAPT duration, showed that a drug-coated stent (polymerand carrier-free biolimus A9-coated stent) was superior to BMS with respect to the primary safety (composite of cardiac death, MI and ST) and efficacy (clinically driven target-lesion revascularisation) endpoints.17
Studies Evaluating Prolonged Duration of Dual Antiplatelet Therapy
The DAPT trial explored the effect of prolonged (30 months) versus 12-month DAPT (clopidogrel or prasugrel) duration in patients with ACS or SCAD at low risk of ischaemic and bleeding events and undergoing stent implantation.4 After first- or second-generation DES implantation, prolonged DAPT significantly reduce the risk of ST, cerebrovascular events and major adverse cardiovascular events. Confirming a general secondary prevention effect of DAPT, much of the benefit shown in the prolonged DAPT group came from a reduction in the rate of MI unrelated to ST. Of particular interest, it was found that overall the rate of ischaemic events increased during the 3-month period after P2Y12 inhibition discontinuation, regardless of when that occurred.4 These data are confirmed by observations from the Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin-Thrombolysis In Myocardial Infarction (PEGASUS-TIMI 54) trial, in which patients with previous MI (39 % with previous DES implantation), enrolled after recent P2Y12 inhibitor withdrawal, were shown to be at increased risk of ischaemic events. This group derived greater benefit in terms of ischaemic risk reduction, from prolonged ticagrelor therapy plus aspirin compared with patients who had remained event-free on aspirin alone.13
Prolonged ticagrelor therapy has also been shown to reduce the rate of major cardiovascular events in patients with a history of MI, regardless of stenting history and stent type, as well as the risk of ST in patients with stents.18 In the DAPT4 and the PEGASUS-TIMI 5413 trials, the benefit in the reduced risk of ischaemic events was accompanied by an increase of Global Use of Strategies to Open Occluded Arteries (GUSTO)-defined moderate/severe bleeding and TIMI major bleeding, respectively. Furthermore, in the DAPT trial a higher mortality rate was observed in patients treated with prolonged DAPT compared with placebo.4 The increased non-cardiac mortality rate with extended DAPT is of uncertain significance and may be explained by an imbalance in the number of patients with cancer diagnosed before the enrolment, reflected in the higher incidence of cancer-related death observed in the prolonged-DAPT group. Consistent with the findings from the DAPT trial on ischaemic outcomes, a post-hoc analysis from the Optimal Dual Antiplatelet Therapy (OPTIDUAL) trial, showed that in patients at low risk of bleeding who underwent DES implantation, there was a trend toward fewer ischaemic events in the group randomised to prolonged (up to 48 months) DAPT therapy with clopidogrel, compared with those who stopped clopidogrel at 12 months, without increased risk of major bleeding.19
Balancing the Risks of Bleeding and Ischaemic Events
Overall, these observations suggest that a 12-month DAPT period does not represent the optimal duration for all patients undergoing a DES implantation, and that the evaluation of DAPT duration should be tailored individually, considering both the bleeding and ischaemic events risk profiles of the patient.15 Individuals at low ischaemic risk, such as patients without ACS who are undergoing PCI, particularly if at high bleeding risk, may be suitable for shortened periods of DAPT, whereas prolonged DAPT (>12 months) could be of more benefit for selected patients without significant bleeding risk or at high ischemic risk, such as patients with previous MI, particularly if presenting with additional cardiovascular risk factors or recurrent ischaemic events. The risk stratification is therefore a crucial step in the decision making regarding DAPT duration. Recently, the DAPT risk score has been presented; this is the first risk score with the advantage of simultaneously assessing both the bleeding and ischaemic risk, thus identifying patients who are likely to derive harm or benefit from prolonged DAPT.20 However, this score was demonstrated in a population free of major bleeding or ischaemic events in the 12-month period of DAPT and should also be validated in other datasets.
Conclusion
To further help clinicians in balancing ischaemic and bleeding risks it is necessary to carry out additional studies, as well as exploring secondary analyses of the most recent trials, to individualise the subgroups of patients that derive the greatest benefit from DAPT prolongation. Until then, clinicians should follow a personalised approach, with an ongoing risk–benefit assessment, rather than a standardised approach.







