Endovascular aortic aneurysm repair (EVAR) was introduced by Volodos in the Ukraine. He attributed much of the concept to Charles Dotter of solid angioplasty fame. The Volodos publication received little attention in the Western world at the time towards the end of the Soviet Union era.1
It was at the 1990 Charing Cross Symposium in London that many in vascular disease management first heard about it. The speaker was the inventor/interventional radiologist, Julio Palmaz. His announcement came as a huge shock to the audience and surgeons looked extremely grim. He described the use of his Palmaz stent to trap and fix a Dacron tube at the neck of an aortic aneurysm, and reported the cases he had done with the surgeon, Juan Parodi, both from Argentina. The subsequent publication by Parodi, Palmaz and Barone reached a huge Western world audience.2 It was then realised that the domain of abdominal aortic aneurysm would no longer be exclusively surgical. High-class catheter skills would be required in future either by learning these or working in harmony with those who have such skills. Unknown to the audience was the work and achievement of breakthrough by Volodos in the Ukraine.
The Endovascular Aortic Repair Trials 1 and 2
Endovascular aortic repair was introduced in London in 1993 and by 1996, the Endovascular aortic repair (EVAR) trials 1 and 2 were designed as separate randomised controlled trials for two different populations of patients.3 EVAR trial 1 was to test EVAR against open repair as reference standard in patients anatomically suitable for EVAR, which proved possible in 54 % of all patients at that time. EVAR trial 1 patients were physically fit enough for open repair from the anaesthesiological point of view and so it was a straight choice between the old method and the new.4
Trials with similar protocols followed in the Netherlands, France and the United States. These trials are the Dutch Randomised Aneurysm Management (DREAM),5 Anévrisme de l’aorte abdominal: Chirurgie versus Endoprothese (ACE)6 and American Outcome following Endovascular versus open Repair (OVER)7 trials, respectively. No substantial disagreements have been reported in these from EVAR trial 1, which was the first to post 10-year results in 2010.4
EVAR trial 2 is quite different and rather surprisingly has never been copied and remains unique. It is therefore the sole trial, which addresses the potential for EVAR in patients in whom the anaesthetic evaluation recommended against open repair at the time. Again, all of the patients who were included in the trial were anatomically suitable for EVAR. The options facing patient and clinician in this instance were either to offer EVAR (possibly under local anaesthesia) or to wait for any improvement in physical state to bring the patient into the range of open repair.8
In EVAR trial 2, patients were randomised to either EVAR with medical care or medical care alone, and results were reported at 30 days, five years and 10 years. The striking finding is how many patients died in association with the poor underlying condition, which prevented open repair (OR) being considered in the first place. Obviously, the associated co-morbidities were the driver here. Against this background, EVAR did not alter the expected day of death. However, it has proved difficult to withhold EVAR in such patients once they are aware that they have a swollen aorta with a natural expectation of bursting. Many seek repair after being given the facts. There is some support for EVAR in the EVAR 2 situation in the per-protocol analysis but the randomised findings are clear overall, where EVAR showed a significantly lower rate of aneurysm-related mortality, but not for all-cause mortality.
For the EVAR 1 trial, aneurysm-related mortality has proven to be a crucial method of evaluating outcome. Sadly again, only EVAR trial 1 uses aneurysm-related mortality whereas all 4 trials, EVAR 1, DREAM, ACE and OVER use all-cause mortality. Early benefit in favour of endovascular against open repair is lost in these trials. For EVAR 1, the absolute aneurysm-related mortality benefit of EVAR is 4 % in the early years but is lost at six years. From that point, the curves run together and stay conjoined in the latest follow-up report.
Long-term Outcome of Endovascular Versus Open Repair
This topic is the rage at international meetings at this moment. This is simply because so much hangs on the late performance of EVAR and what will happen next. Loud voices from the podium preach reassurance in terms of EVAR. This is understandable, as patients prefer EVAR. It is estimated that in the US, approximately 75 % of patients receive EVAR ahead of OR, and also in many other countries, certainly in Europe, EVAR is well ahead in terms of procedures reported. Huge research and development has gone into devices, and excellent training is available to assure optimal results the world over. However, it is facts that we need and that we must have to safeguard our patients and do all in our power to maintain optimal long-term results from aortic aneurysm repair.
Factors to Determine the Ultimate Outcome of Endovascular Against Open Repair
There are three factors, which can affect outcomes. Our inclination is towards meeting these problems head-on rather than assuming all is in order and that there is no problem in the long-term outcome of EVAR. It is likely that these factors will be the key to long-term success. The crucial results will come from long-term randomised controlled trials, which provide the highest level of evidence and will influence practice. Registries provide helpful collaborative data but there is always a problem in terms of whether the series is consecutive and if entry to it is properly defined at the outset. It is fairly useless to have large cohorts of registry data from sources never intended for EVAR follow-up or where such crucial information as pre-operative aortic diameter was never recorded. It is also unfounded to say that ‘real-life’ data are superior if by ‘real life’ is meant a registry of today recording results on smaller aneurysms and followed only for a few months. To compare such with randomised controlled trials is a misjudgement as the latter were consecutive, prospective and for a known indication and size of aneurysm. Thus, the answer has to come from trials long under way and the focus has to be on long-term outcomes of up to 15 years.
Reinterventions and Endoleaks
During the follow-up of EVAR, computerised tomography (CT) has been the mainstay. In the EVAR trials, long-term follow-up is by annual CT scan. There is an understandable trend towards ultrasound after the 10-year EVAR trials results were published. This is driven mainly by cost considerations and patient convenience. Endoleaks have been detected during EVAR follow-up and these have required reintervention, especially when considered dangerous. A core-laboratory was set up using a validated three-dimensional workstation (Vitrea 2, Version 4.3.044.0, Vital Images Inc., Minnetonka, MN, US) to process all available decent-quality CT scans of the EVAR trials.9 An audit was performed and published on those EVAR devices, which were reported to have suffered secondary sac rupture. Of note, these ruptures were associated with a 67 % mortality.10
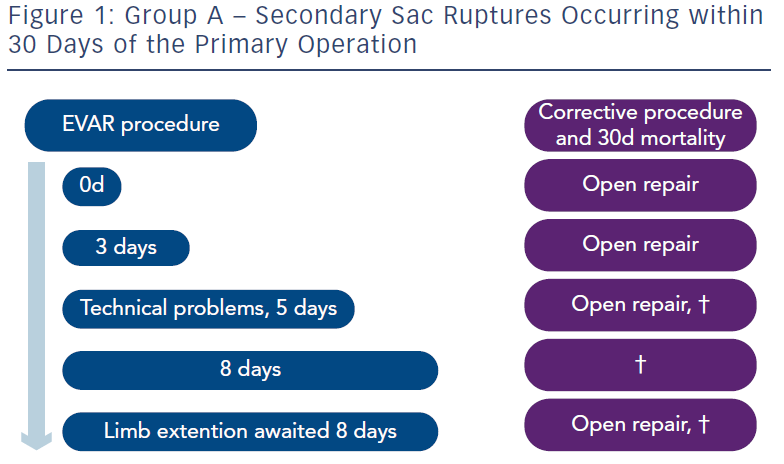
Three groups were described by the authors. In Group A there were five patients who suffered rupture before 30 days (see Figure 1). These ruptures would have been avoided if a CT scan had been performed before hospital discharge. Some who hear these results have alleged “any fool should know that”. It is regrettable that pioneers of these procedures who submitted their practice to a very early randomised controlled trial did not know this in 1999 when the trials began. Instead, when an EVAR procedure was nearing completion, a final flush angiogram would be performed and, if satisfactory, patients were discharged very soon and in some cases on the very same day. We are not referring to individual practice but this happened partly to show that it could be done. Whether it should be done was always another matter. But a CT scan would have detected in all probability a dangerous situation, which needed correction before discharge from hospital. This soon became accepted practice.
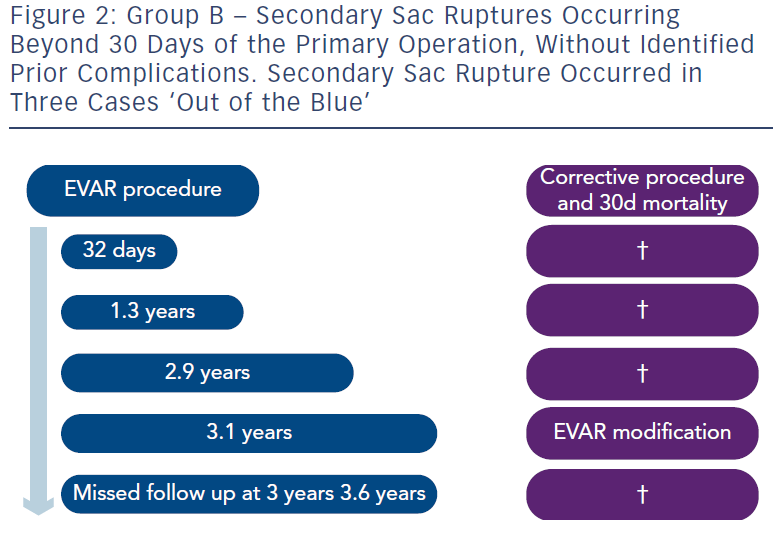
In Group B were five patients who suffered rupture after 30 days and without known endoleaks. Three of these were ‘out of the blue’ (see Figure 2). One was at 32 days and really like Group A. The fifth refused proper follow-up and suffered fatal rupture.
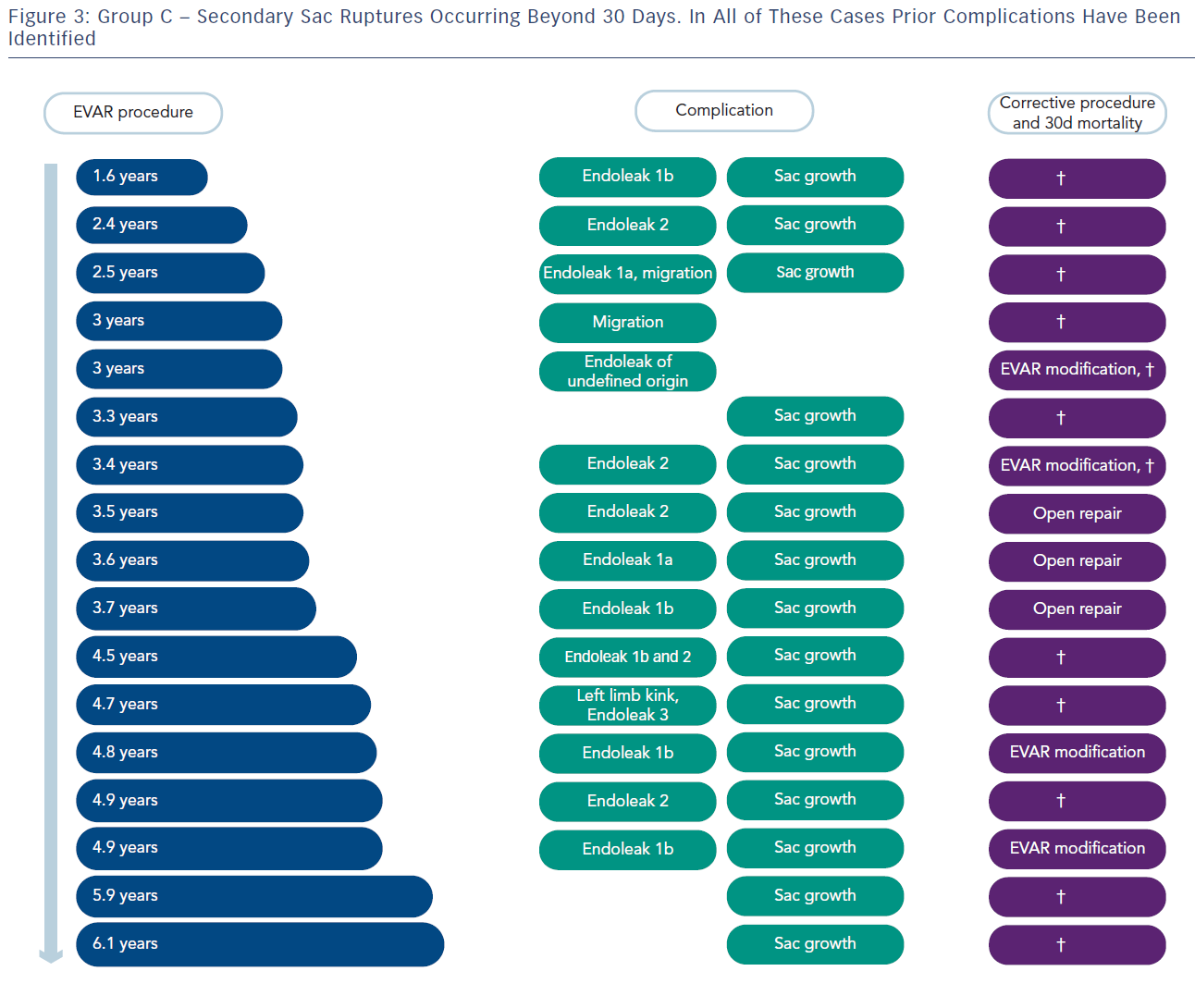
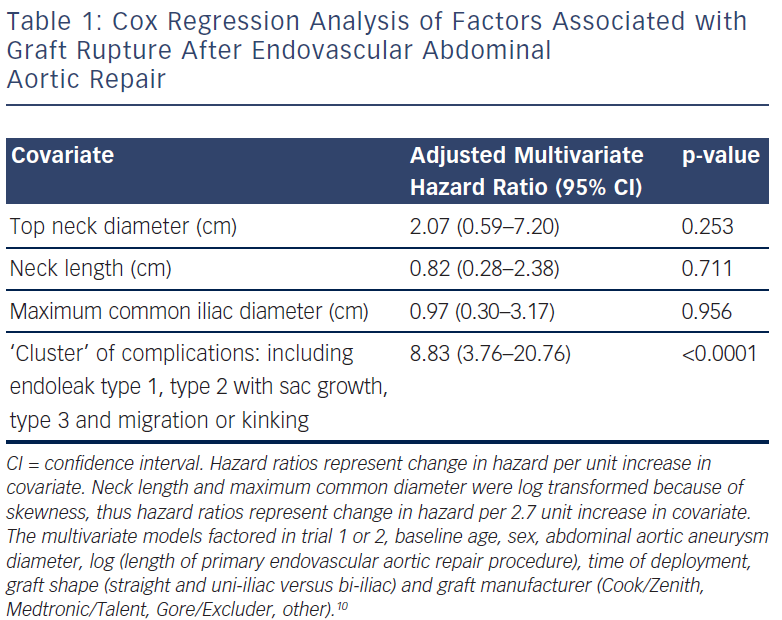
Group C had 17 patients and these had sac rupture but with known underlying endoleaks (see Figure 3). A dangerous ‘cluster’ was described of types 1 and 3 endoleaks, migration, kinking and type 2 endoleak with sac expansion.10 Fifteen of the 17 had sac expansion and what has been referred to by Gilling-Smith as endotension, although that term is a concept rather than a measurement of pressure in the sac.11 It is visualised that an endoleak leads to expansion of the sac and rupture. Migration and kinking with poor endograft fit to the aorta is conceptually predisposed to dangerous endoleaks. These factors as a ‘cluster’ were fed into the statistical analysis of all EVAR patients in trials 1 and 2 and there was a statistically significant correlation (see Table 1)10. The ‘cluster’ denotes a dangerous finding in the late follow-up of EVAR.
Later Generation Endograft Devices
Exciting new devices are appearing that are especially designed to improve late performance of EVAR. It is very encouraging that this is being addressed so widely. Huge resources are going into this. Key to advances is the attention paid to improved conformability to the aortic neck and to the iliac landing zones. The potential weaknesses of the methodology are taken on board and newer devices orientate towards this. Kinking of endograft at the aortic neck may occur as a result of angulation of the neck and the same applies to the iliac landing zone. The more recently available devices conform to an aortic shape better and it is to be expected and hoped that this will translate into improved long-term results. In principle, the newer devices should reduce endoleak in the late stages and so reduce secondary sac expansion and subsequent rupture, but this is speculative and unproved at this point.
Migration of endograft has been addressed by certain manufacturers for years. Hooks and barbs are by no means applied by all manufacturers and neither is suprarenal fixation. All manufacturers are nevertheless completely mindful of the need to avoid endograft downward movement and with this the risk of type 1 endoleak.
Easier deployment with more reliable placement and the ability to adjust position is another very helpful improvement. There is an inevitable move towards percutaneous deployment introduced to avoid groin wound problems. Various sealing methods improve these techniques but the drift towards lower profile of endografts needs to be monitored carefully to be sure that the thinner and flimsier materials are as durable. Otherwise late failure becomes an increased risk.
All in all, the newer devices should improve EVAR late results and reduce secondary sac rupture but the most rigorous follow-up is required to track this.
Abuse of Instructions for Use
Roy Greenberg’s group has drawn attention to this.12 It emerges that as endograft deployment has gathered ground and clinicians have become more confident, EVAR is preferred even outside the instructions for use.
This is less a comment on deployment for aneurysm of less than 5.5 cm than a concern that EVAR is expected by some to cope with aortic anatomies, which lie beyond the reliable compass of EVAR.
The UK Small Aneurysm Trial (UKSAT)13 and The Department of Veterans Affairs Aneurysm Detection and Management (ADAM)14 trials have made a global impact upon discouragement of unnecessary correction of abdominal aortic aneurysm of less than 5.5 cm yet still a vast number of these procedures is performed.12 There remains no evidence to support such practice.
Of increasing concern is that a somewhat ‘cavalier attitude’ to EVAR deployment is creeping in, which is driven by a type of machismo approach to demonstrate ability to fix any aorta with EVAR. Certainly the patients prefer it but would they prefer the method if it is explained to them that the proposed procedure is beyond the manufacturer’s instructions for use and that late failure could be an increased risk? Of course, the experienced operators should and must push the boundaries but each individual operator needs to be mindful of the risks of late EVAR failure.
Summary
Three factors will determine the long-term success of EVAR or the resurrection of the open surgical method for younger and physically fit patients.
First, there is the urgent need to correct the ‘cluster’ of dangerous endoleaks and this implies ongoing careful surveillance. If CT has given way to ultrasound, it is because that method is very reliable especially in measuring front to back maximal infrarenal aortic diameter. However, if either endoleak is suspected or an increase of 5 mm sac diameter occurs in a year, CT is required to reveal any potential underlying endoleak, migration or true sac diameter increase and the reason for it. A renewed effort to search for this ‘cluster’ is mandatory. This ought to affect late EVAR results by reducing late sac ruptures. Second, the availability of the excellent latest devices should help to reduce late complications. However, thirdly, an inclination to use EVAR beyond instructions for use by the less expert performers could put the long-term results of EVAR into question. Full training in EVAR deployment continues to be of the utmost importance, however, open surgical expertise and catheter skills are equally required. It has to be recognised that in some situations, an open repair is not a failure in clinical practice but the best way forward for a particular patient with unfavourable anatomy.