Transcatheter aortic valve implantation (TAVI) is now established as the standard treatment for symptomatic severe aortic stenosis (AS) in patients at high or prohibitive surgical risk, and the preferred treatment for those at intermediate risk.1
Based on recent trials in low-risk patients, the indications for TAVI are expanding towards the lower-risk classes, and the procedure has even been discussed for younger and asymptomatic patients.2,3 Although a significant reduction in periprocedural morbidity and mortality has been observed for TAVI over the past decade due to better patient selection, device design and operator experience, the occurrence of periprocedural conduction disturbances remains a concern.4
The most common post-TAVI conduction abnormalities are left bundle branch block (LBBB) and high-degree atrioventricular block (HAVB) requiring pacemaker implantation (PMI).5 Although the clinical effects of new-onset LBBB and PMI after TAVI remain controversial, substantial evidence supports an association of these conduction abnormalities with adverse effects.6,7 With an expanding indication for TAVI, the possible deleterious consequences of LBBB and PMI need to be taken into account and further clarified. We review the current evidence in conduction abnormalities after TAVI and how to manage them.
Mechanisms of Conduction Disturbances in Transcatheter Aortic Valve Implantation
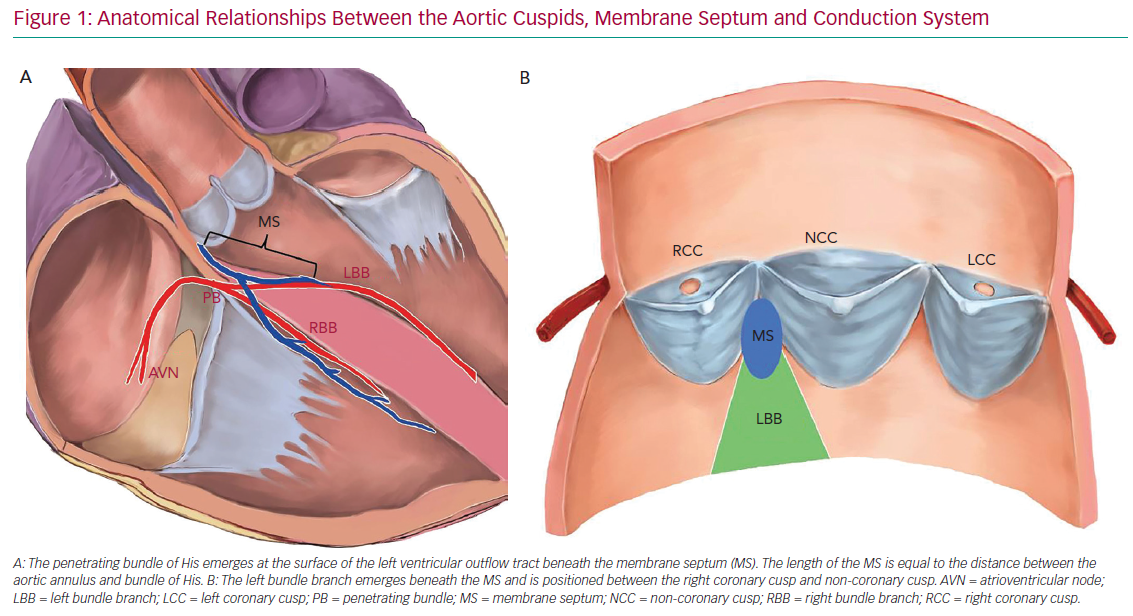
The development of periprocedural conduction disturbances can be explained by the proximity of the aortic valve with the conduction system. The atrioventricular (AV) node is located within the triangle of Koch in the right atrium and is in close proximity to the subaortic region and the membranous septum (MS). The AV node continues as the bundle of His and branches into the right and left bundle branch (RBB and LBB, respectively). The bundle of His is located in the MS and the LBB emerges at the level of the non-coronary aortic cusp, just below the posteroinferior MS edge. Thus, the length of the MS is equal to the distance between the aortic annulus and the exit point of the bundle of His.8 This close relationship between the LBB and aortic cusp explains the predisposition to conduction disturbances after TAVI (Figure 1).
Direct mechanical insult to the conduction system due to inflammation, oedema, localised haematoma and ischaemia of surrounding tissue caused by TAVI was demonstrated by previous studies.9 If a branching bundle of His lies superficially within the left half of the ventricular septum, or the LBB is briefly exposed, the chances of conduction disturbance after TAVI are increased.10
New-Onset Left Bundle Branch Block After Transcatheter Aortic Valve Implantation
Incidence
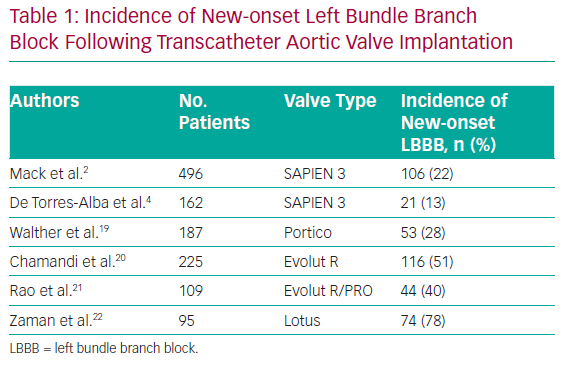
New-onset LBB block (LBBB) is the most frequently observed conduction abnormality after TAVI. The incidence of new-onset LBBB varies considerably in previous studies because of differences in the inclusion of transient LBBB, timing of measurement and the type of transcatheter valve. The incidence of new-onset LBBB has been reported to range from 4% to 65% after TAVI with first-generation valves.11–14 The incidence of new-onset LBBB was higher with self-expandable CoreValve devices (Medtronic), with rates ranging from 18% to 65%, than with balloon-expandable SAPIEN/SAPIEN XT devices (Edwards Lifesciences), with rates ranging from 4% to 30%.15
The incidence of new-onset LBBB after TAVI with newer-generation devices ranges from 13% to 78% (Table 1). Similar occurrence rates were reported for SAPIEN 3 (13–22%) and Portico (Abbott Vascular; 28%).2,4,16–19 The incidence of new-onset LBBB appears to be more frequent for some self-expandable newer-generation devices, such as the Evolut R/PRO (Medtronic; 44%) and Lotus (Boston Scientific; 74%).20–22
Timing
Most of the conduction disturbances after TAVI occur periprocedurally or within the first 24 hours of TAVI, with 90% diagnosed within the first week after the procedure.23 New-onset LBBB can be transient and recover within the first few days. In a previous study, only 52% of patients who developed new LBBB after CoreValve implantation had persistent LBBB at discharge, but this mostly persisted out to 30 days.24 A recent study showed new LBBB after TAVI resolved in 33% of patients at 1-year follow-up, and no clinical or ECG variables predicted LBBB recovery.25 Late-recovery or delayed-onset LBBB after discharge or at 30 days is rare. Delayed-onset LBBB at discharge to 30 days was reported in only 1.8% of patients after SAPIEN valve implantation and in 2.9% of patients at 6 months to 1 year.13
Clinical Outcomes
A previous study reported that new-onset LBBB worsened 1-year survival after surgical aortic valve replacement.26 However, the clinical impact of new-onset LBBB after TAVI remains controversial. De Carlo et al. reported that of 275 patients undergoing TAVI, 34.5% developed new LBBB.27 Among patients who did not undergo PMI, 1-year overall survival rates were similar between those who developed new LBBB and those who did not.27 Similar results were reported among 201 Asian patients undergoing TAVI; new-onset LBBB or PMI was not associated with 1-year all-cause and cardiovascular mortality, or with hospitalisation due to heart failure.28 Moreover, in a meta-analysis of 4,756 patients, new-onset LBBB was not associated with a significant increase in all-cause mortality.29 However, that study reported that new LBBB was associated with a higher risk of 1-year cardiovascular mortality and PMI. Data from the Placement of AoRTic TraNscathetER Valve (PARTNER) Trial also showed that new-onset LBBB was associated with increased PMI during hospitalisation (8.3% versus 2.8%; p=0.005) and from discharge to 1 year (4.7% versus 1.5%; p=0.01).13 In addition, left ventricular ejection fraction (LVEF) declined more and was significantly lower in patients with than without new LBBB (53.4% versus 57.4%; p=0.02).
Conversely, some studies have demonstrated that new-onset LBBB is an independent predictor of all-cause mortality at more than 2 years of follow-up.30,31 Nazif et al. reported the analysis of 1,179 intermediate-risk patients from the PARTNER II trial and revealed that new-onset LBBB was associated with increased all-cause mortality, cardiovascular mortality, rehospitalisation and new PMI at the 2-year follow-up.32 Similarly, another recent study reported that new-onset LBBB did not increase the risk of long-term all-cause mortality, cardiovascular mortality or rehospitalisation at a median follow-up of 3 years; however, it was associated with higher in-hospital mortality, PMI and lack of LVEF improvement.20 The discrepancy between studies may be explained by differences in patients’ baseline risk, sample size, type of transcatheter heart valve used, application of diagnostic ECG criteria, absence of a standardised definition of new-onset LBBB and the duration of follow-up.
A recent meta-analysis of 12 studies summarised the clinical impact of new LBBB and reported an increased risk of all-cause death at 1-year follow-up in patients with new LBBB (RR 1.32; 95% CI [1.17–1.49]; p<0.001].33 It also demonstrated that the presence of new LBBB after TAVI was associated with a higher risk of 1-year cardiac death (RR 1.46; 95% CI [1.20–1.78]; p<0.001), 1-year heart failure hospitalisation (RR 1.35; 95% CI [1.05–1.72]; p=0.02) and 1-year PMI (RR 1.89; 95% CI [1.58–2.27]; p<0.001).
Management of New-onset Left Bundle Branch Block
There are no standard guidelines to manage new-onset LBBB after TAVI. Because of the potential risk of early progression to HAVB (7–16%), monitoring patients with new LBBB with telemetry or daily 12-lead ECG for at least 2 days seems reasonable. Auffret et al. suggested keeping the temporary pacemaker and monitoring those patients with new LBBB in an intensive care unit for at least 24 hours.34 There is still no effective measure to manage patients with new-onset LBBB that lasts 48 hours after TAVI. In a study of 3,726 patients who had undergone TAVI, new-onset LBBB and QRS duration >160 ms at discharge were associated with increased risk of sudden cardiac death.35 Therefore, prophylactic PMI in this setting may be reasonable. A recent study reported that 9% of patients with new-onset LBBB developed advanced conduction disturbances requiring PMI during a 1-year follow-up period.25 In that study, the presence of AF, a longer PR interval at discharge and a longer PR interval change between baseline and discharge were associated with an increased risk of PMI. Close monitoring (i.e. Ziopatch) in this group of patients may be reasonable. Almeida et al. studied a cohort of 138 patients after TAVI using multidetector CT (MDCT) and demonstrated that implantation depth assessed by MDCT is associated with new-onset conduction disturbances after TAVI.36 Postprocedural MDCT has the possibility of detecting patients at high risk of late-onset conduction disturbances.
A multicentre prospective study evaluated the role of an implantable cardiac monitor during the first year of follow-up in 103 patients with new-onset LBBB after TAVI.37 Significant bradycardia events were reported in 20% of patients and HAVB was observed in 15% of patients. PMI was required in 10% of patients.37 These data support the use of a cardiac monitor device for close follow-up in this group of patients; however, the level of evidence for this measure remains low, and more evidence is still needed to determine which patient profile will benefit more from a prophylactic PMI approach.
Rodés-Cabau et al. recently proposed a strategy algorithm for the management of patients with new-onset LBBB after TAVI.38 Some characteristics of patients at higher risk were addressed and more aggressive management was recommended. Patients with persistent LBBB at Day 2 with QRS ≤150 ms and PR ≤240 ms could be discharged and continuous ECG monitoring (2–4 weeks) could be considered. Patients with persistent LBBB at Day 2 with QRS >150 ms or PR >240 ms were at increased risk of delayed HAVB requiring PMI, and continuous ECG monitoring or electrophysiology studies may be considered to guide PMI decision. If further prolongation of the QRS or PR interval (of at least 20 ms) was observed after 24 h, evaluation with electrophysiological studies (followed by continuous ECG monitoring if no PMI) or direct PMI may be considered.
Pacemaker Implantation After Transcatheter Aortic Valve Implantation
Incidence
Conduction disturbances requiring PMI are the most common complications after TAVI. A meta-analysis reported that among 11,210 patients from 41 studies, 1,917 (17%) underwent PMI after TAVI.39 The incidence of PMI after TAVI ranged from 2% to 51% in individual studies. Similar to new-onset LBBB, PMI is more frequent with the first-generation Medtronic CoreValve than with the Edwards SAPIEN transcatheter heart valve.39,40 An Italian national prospective observational study comparing five leading new-generation TAVI devices reported that the incidence of PMI ranged from 5.6% to 23.2%.41 The five new-generation devices included ACURATE (Boston Scientific), Evolut R/PRO, Lotus/Lotus Edge (Boston Scientific), Portico and SAPIEN 3/SAPIEN 3 Ultra, with the results favouring ACURATE, with the Lotus transcatheter heart valve being associated with a higher rate of PMI. A registry enrolling 1,000 patients undergoing TAVI using a self-expanding ACURATE Neo showed a low rate of new PMI (9.9%), which is lower than rates for SAPIEN 3, Evolut R/PRO and Lotus.21,42–44
Several studies have reported that periprocedural TAVI complications have been significantly reduced with the introduction of newer-generation devices; however, no marked improvement in terms of PMI rate has been reported.45–47 Some studies showed an increased rate of PMI with SAPIEN 3 compared with the older SAPIEN XT (13.6% versus 9.5%; p=0.001).47 In addition, patient surgical risk does not seem to affect the rate of PMI. A multicentre Australian cohort reported that PMI rates were similar among patients with different classes of risk (21%, 27% and 26% in low-, intermediate- and high-risk groups, respectively).48 Conversely, a recent meta-analysis encompassing three randomised studies comparing TAVI to surgical aortic valve replacement in low-risk patients showed that, at 1 year, TAVI was related to a higher PMI risk (RR 3.47; 95% CI [1.33–9.07]; p=0.01).49
Some limitations regarding the evaluation of the incidence of PMI should be considered. Although HAVB is the most common indication for PMI after TAVI, the reported indications for PMI were inconsistent across the studies and may vary according to the operator or hospital criteria, with some institutions having a more aggressive approach than others. Therefore, the proportion of patients who have undergone ‘prophylactic’ PMI not following the current guidelines is unknown. In addition, the current trend of shorter postprocedural hospital stay is a factor that can interfere with prophylactic PMI indications, resulting in a shorter period of clinical observation for new-onset conduction disturbances.
Timing and Evolution of High-degree Atrioventricular Block Leading to Pacemaker Implantation After TAVI
Similar to new-onset LBBB, TAVI-induced HAVB occurs primarily in the periprocedural phase. A previous study showed that in patients requiring PMI due to HAVB after TAVI, 87% of HAVB occurred in the periprocedural phase.50 Toggweiler et al. studied 1,064 patients undergoing TAVI with CoreValve or SAPIEN XT/SAPIEN 3: 163 patients had new-onset of HAVB, of whom 56% had periprocedural HAVB and 44% had delayed HAVB (defined as not present on the first ECG after TAVI but occurring during 30-day follow-up).51 Among those with delayed HAVB, most events occurred within the first 48 hours and only 2.3% occurred 3–8 days after TAVI. Several lines of evidence support that the risk of late-onset conduction disturbance is low in patients with a normal ECG after TAVI. Independent predictors for late-onset conduction disturbance requiring PMI include pre-existing non-specific intraventricular conduction delay, pre-existing RBB block (RBBB), self-expandable valves and predilation.52
Most HAVB tends to recover over time. In a study with 234 consecutive patients who underwent TAVI with CoreValve, 27.4% of patients underwent HAVB-related PMI. Half the patients who had an absolute indication for PMI had resolution of the conduction abnormality after 24 hours after TAVI.53 In another study of 1,198 patients who underwent TAVI, only 22.4% of patients who developed HAVB requiring PMI had persistent complete heart block at a follow-up of 73 days.54 Miura et al. provided long-term data on interrogations of the implanted pacemaker due to HAVB after TAVI with balloon-expandable valves: at 6 months and 1 year after PMI, 60% of patients who recovered from bradycardia had a ventricular pacing rate of ≤1.0%.28
Clinical Outcomes
Right ventricular (RV) pacing results in inter- and intraventricular desynchrony, with subsequent detrimental effects on cardiac structure and function. Evidence supports RV pacing causing chronic left ventricular (LV) remodelling and, in some cases, possibly leading to adverse clinical outcomes, such as AF, heart failure and death.55–57 However, the clinical impact of PMI after TAVI remains controversial.
A meta-analysis including 7,032 patients reported that periprocedural PMI after TAVI was not associated with an increased risk for all-cause mortality at 1 year.29 In that study, a potentially protective effect of PMI on 1-year cardiac death was observed (RR 0.77; 95% CI [0.58–1.01]; p=0.06) in 4,362 patients following TAVI. This protective effect may be explained by PMI preventing progression towards complete AV block and sudden death. Another multicentre study including 1,629 patients undergoing TAVI reported that 19.8% of patients required PMI.21 After a median follow-up of 4 years, PMI was associated with an increased risk of heart failure rehospitalisation and a lack of LVEF improvement; however, there were no differences in all-cause and cardiovascular mortalities between those with and without PMI.21
In contrast, a negative clinical impact was reported in a cohort of 9,785 patients who underwent TAVI.58 PMI was required in 6.7% after TAVI and was associated with longer lengths of stay in hospital and in the intensive care unit. PMI was also related to higher mortality (24.1% versus 19.6%) and a composite of mortality or heart failure admission (37.3% versus 28.5%) at 1 year.58 A recent study reporting long-term follow-up results in a cohort of 1,116 post-TAVI patients showed that PMI was indicated in 13% of patients.59 At the 6-year follow-up, PMI was associated with increased all-cause mortality (57.0% versus 41.7%; p=0.034).59 Faroux et al. conducted a meta-analysis including 21 studies and demonstrated a deleterious effect of PMI on all-cause death (RR 1.17; 95% CI [1.11–1.25]; p<0.001) at the 1-year follow-up.33 That study also showed that PMI increased the risk of heart failure hospitalisation at 1 year (RR 1.18; 95% CI [1.03–1.36]; p=0.02), but had no significant effect on cardiac death at 1 year (RR 0.84; 95% CI [0.67–1.05]; p=0.13).
The negative effect of RV pacing is related to the percentage of pacing. Only a few studies have reported pacemaker pacing frequency after TAVI; therefore, the clinical impact of PMI becomes hard to demonstrate. In a study of pacemaker dependency after TAVI, Costa et al. reported pacemaker dependency rates of 35.7%, 35.8% and 33.3% at 1, 6 and 12 months, respectively.59 At 6 years, pacemaker-dependent patients showed a higher overall mortality than non-dependent patients. Conversely, the negative effect of chronic pacing is counterbalanced by the protective effect of PMI against the risk of sudden death. Moreover, most prior studies enrolled TAVI patients who were elderly and vulnerable; thus, reduced life expectancy may limit the appearance of clinical outcome due to chronic pacing-related ventricular dysfunction.
Management of High-degree Atrioventricular Block
The latest European Society of Cardiology guidelines recommend that a period of clinical observation up to 7 days is indicated in order to assess whether HAVB after TAVI is transient and resolves before patients undergo PMI.60 This recommendation is supported by the observation that a significant proportion of HAVB recovers over time. Considering the potential negative clinical impact of PMI after TAVI and complications associated with pacemakers, such as lead-related damage, pneumothorax, pocket haematoma or pacemaker infection, it is reasonable to have a watchful period before PMI.21,58,59 Conversely, a prolonged observation period with temporary pacemaker increases the risk of patient immobilisation, thromboembolism, catheter-related infection and cardiac perforation.61
However, prior studies showed that most PMI after TAVI occurred within the first 5 days. One reason is the current trend toward minimalist TAVI and the early discharge strategy. A meta-analysis of 1,775 patients comparing clinical outcomes between those with early (≤3 days) and standard discharge after TAVI found no significant differences between the two groups in terms of 30 day mortality and new PMI rates.62 This suggests that a watchful period of 3 days after an HAVB episode may be sufficient in selected patients. Auffret et al. suggested monitoring patients in intensive care units and keeping the temporary pacemaker for 24–48 hours before patients undergo PMI.34 Rodés-Cabau et al. proposed a comprehensive strategy of HAVB management and suggested that a 24-hour observation period following the procedural HAVB episode was a reasonable compromise.38 If HAVB persisted at 24 hours after TAVI, PMI was recommended and, if HAVB recovered, the temporary pacing wire could be removed with telemetry and daily ECG monitoring for one more day. If another episode of HAVB occurred during the 24-hour period, PMI was recommended.38 Patients could be discharged at day 2 after TAVI if there were no other episodes of HAVB and no other features potentially justifying PMI.
Predictors of Conduction Disturbances After Transcatheter Aortic Valve Implantation
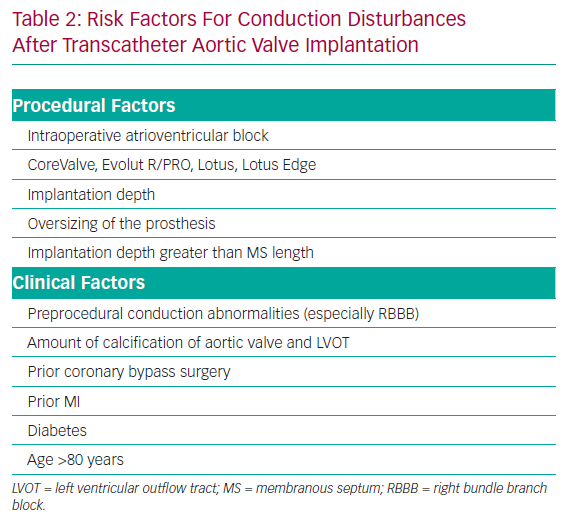
Previous studies reported that predisposing factors for conduction disturbances after TAVI include preprocedural conduction abnormalities, previous coronary artery bypass surgery, diabetes, severity of calcification on the aortic valve, implantation depth, larger valve size, degree of prosthesis overexpansion and the use of a CoreValve system (Table 2).31,63–67
A meta-analysis reported that CoreValve implantation was associated with a 2.5-fold higher risk of PMI, which can be attributed to the greater radial force that exerts significantly more mechanical stress on the conduction system than a balloon-expandable prosthesis.39 Pre-existing RBBB was also reported as a strong independent predictor of PMI after TAVI;39 this can be explained by the implanted prosthesis primarily affecting the LBBB and leading to complete AV block when there is a predamaged RBB. Valve oversizing by 10–15% has been associated with a higher risk of PMI after TAVI with first-generation devices.68 The MS length, a surrogate for the distance between the aortic annulus and the penetrating bundle of His, has been identified as a predictor of HAVB and PMI.8 A recent study demonstrated that there was higher risk of HAVB after TAVI when the implantation depth was greater than MS length rather than considering implantation depth alone.69 Kiani et al. reported on a cohort of 1,266 patients who underwent TAVI with SAPIEN 3.70 In that study, the Emory risk score was proposed and validated on the basis of PMI risk predictors: a history of syncope (1 point), QRS duration ≥138 ms (1 point), pre-existing RBBB (2 points) and a degree of valve oversizing ≥16% (1 point). Patients with higher risk scores were more likely to require PMI after TAVI.70
Strategy to Prevent Pacemaker Implantation
Preprocedural evaluation is important to prevent PMI. The presence of predisposing factors for PMI (Table 2), including baseline RBBB, may inform the need for careful procedural planning and continued observation for conduction deficits after the procedure.
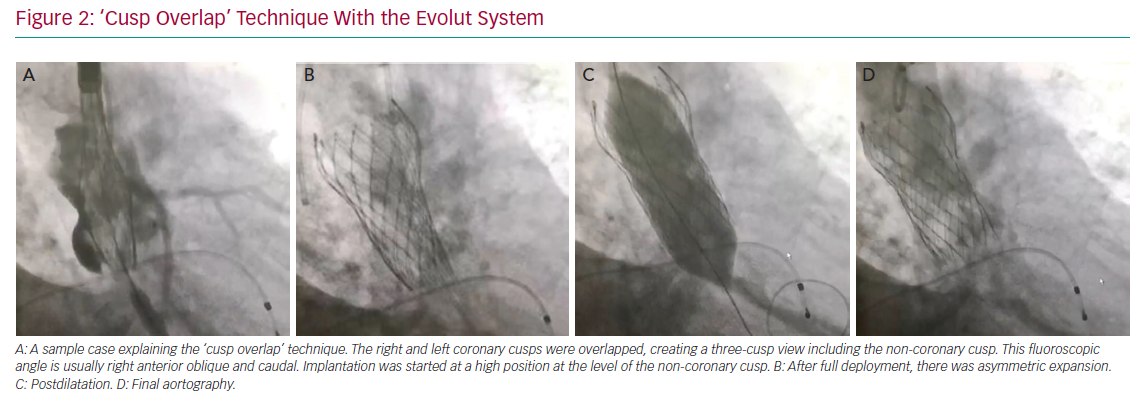
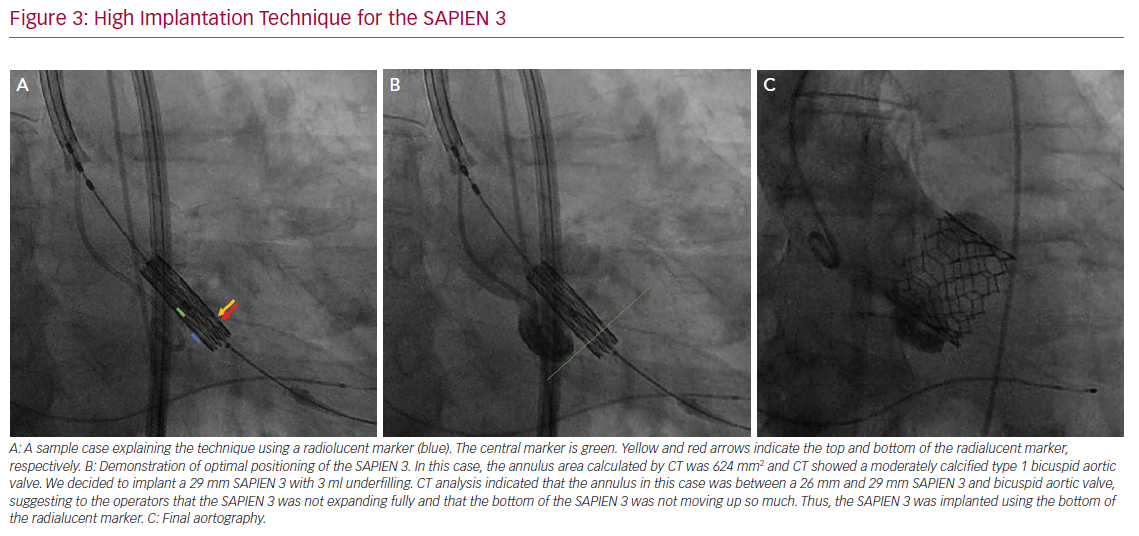
The variables that can be controlled during the procedure are the depth of implantation, the choice of a self- or balloon-expandable valve and avoiding an oversized valve. Jilaihawi et al. suggested using an anatomically guided approach for device positioning based on the CT-determined MS length.69 This minimally higher depth of implantation approach reduced the rate of new PMI after placement of a self-expandable valve to only 3% in that study cohort. In terms of the procedure itself, Tang et al. suggested using the ‘cusp overlap’ fluoroscopy view to guide implantation of self-expandable valves.71 A coplanar projection by overlapping the right and left coronary cusp offers several advantages: the delivery catheter more centred across the aortic valve and an en face view of non-coronary cusp (NCC) enable higher valve implantation with a lower risk of device embolisation (Figure 2). In addition, some centres have recently tried to perform high implantations using the radiolucent marker of the SAPIEN 3 (Figure 3a, blue line) instead of centre maker (Figure 3a, green line). In the case of nominal volume or overfilling, the top of the radiolucent marker was used to align the bottom of the NCC (Figure 3a, yellow arrow) and implant the SAPIEN 3. In the case of underfilling, the bottom of the radiolucent maker (Figure 3a, red arrow) was used to align the bottom of NCC and implant the SAPIEN 3. With this strategy, higher implantation is achieved (Figures 3b and 3c), which minimises the rate of PMI.
Choice of Pacemaker
New LBBB after TAVI is associated with a decline in LV function, particularly in patients with pre-existing LV dysfunction.13 Biventricular pacing was demonstrated to have a beneficial effect on patients with HAVB and LV dysfunction.72 Previous case reports showed resynchronisation therapy improved the LV function of patients with chronic LV dysfunction developing persistent LBBB after TAVI.73,74 This strategy seems reasonable in select cases, but more evidence is needed to confirm the timing of implantation and potential clinical impact.
A previous study suggested that periodic examination and adjustment of pacemaker settings minimised the risk of long-term pacing in patients requiring PMI after TAVI.75 Prior studies also showed pacemaker dependency after TAVI was not so high.28,59 Thus, leadless pacemakers may contribute to less morbidity in these patients. Currently there is little evidence concerning the use of leadless pacemakers in patients after TAVI. A case report described the leadless pacemaker as an alternative choice for patients with conduction disturbances after TAVI to minimise procedural-related damage, especially for old and frail patients.76
Conclusion
Conduction disturbances remain a challenge even in the contemporary TAVI era. Most common conduction disturbances are new-onset LBBB and HAVB requiring PMI. These complications may portend a negative clinical impact on patients. Several predictors of conduction disturbances after TAVI have been reported previously. Efforts should be made to mitigate the risk of conduction disturbances after TAVI by considering preprocedural risk assessment, periprocedural planning, device selection and implantation technique. Further prospective studies are needed to define the optimal watchful period before PMI and to identify specific patients who may benefit from prophylactic and leadless PMI.