Aortic stenosis (AS) is one of the most common valvular diseases in developed countries and its prevalence is projected to increase over the next decade as the population ages.1,2 Transcatheter aortic valve implantation (TAVI) is an established alternative to surgery for treatment of patients with symptomatic, severe AS.3
As the latest multicentre trials have established the role of TAVI alongside surgery for treatment of lower-risk patients, it will be offered to younger people in the upcoming years.4,5 In this particular subset, life expectancy is expected to exceed that of the initial high-risk candidates for TAVI, which were older and had multiple, severe comorbidities. This makes it crucial to gather data on the durability of long-term transcatheter valve prosthesis.
The lack of standard definitions of structural valve degeneration (SVD) had made it difficult to compare studies on the durability of surgical or transcatheter bioprostheses due to the high heterogeneity of adopted criteria.6–11 Since the release of standardised SVD definitions by the European Association of Percutaneous Cardiovascular Intervention (EAPCI), the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS), an increasing number of studies have reported encouraging outcomes after TAVI with either balloon- and self-expanding transcatheter aortic valves (TAV) up to 7 and 8 years. In cases of bioprosthesis failure due to SVD, redo TAVI has been shown to be a reliable alternative to redo surgery. This article will examine the emerging issue of TAV degeneration and its management.
Causes and Mechanisms of Bioprosthesis Degeneration
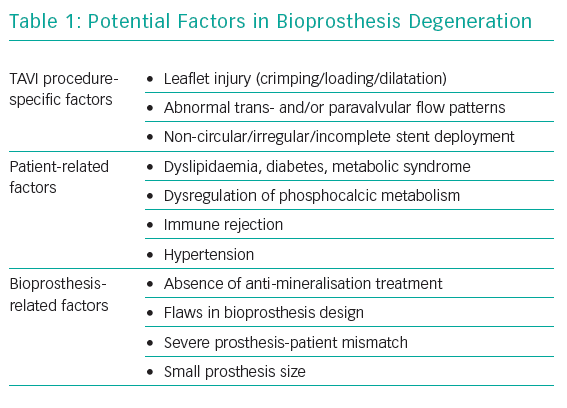
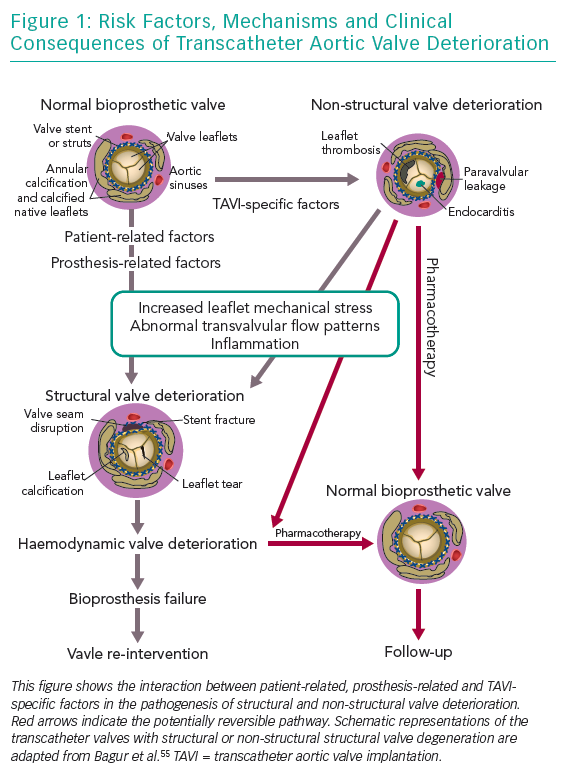
Bioprosthesis degeneration can be related to different aetiologies, including leaflet degeneration, endocarditis, thrombosis and paravalvular leak (Table 1 and Figure 1).
Leaflet Degeneration
The main mechanism behind bioprosthesis degeneration is leaflet degeneration. This happens in a similar way as for the native aortic valve and consists in a step-wise process leading to tissue calcification and consequent valve stenosis or regurgitation.12 Although TAVs demonstrated to degenerate in a manner comparable to surgical bioprostheses, three key differences between TAV and surgical aortic valve (SAV) degeneration mechanisms have been identified:13
- The presence of turbulence in the aortic root due to the combined presence of bulky calcium nodules in the sinuses of Valsalva and the prosthetic aortic valve. This may affect blood flow, resulting in chronic mechanical stresses on the prosthetic valve leaflets, leading to early degeneration.
- The trauma that may occur to prosthetic valve leaflets when loading into a delivery catheter before being established in its anatomical position.
- After deployment, TAVs, particularly the self-expanding ones, often have an oval shape which could affect the normal valvular functioning. Native aortic cusps calcifications, the conformation of the left ventricular outflow tract (usually non-circular) and prosthetic valve oversizing (for reducing paravalvular leakage) lead TAVs to become non-circular, which could affect long-term durability, although the real impact is not known.14
Endocarditis
Infective endocarditis has been reported to occur in 0.5–3.4% of cases after TAVI and 1–6% after surgical aortic valve replacement (SAVR).13,15 The predominant agents reported to have caused prosthetic valve endocarditis were Staphylococci (31.5%), Enterococci (20%) and Streptococci (14%), based on 29 certain cases of endocarditis from about 4000 TAVI patients of a multicenter registry.15 Diagnosis of TAV endocarditis can be difficult as older patients may present with subtle and atypical symptoms.16 Endocarditis is shown with echocardiography as mobile vegetations, aortic root abscess formation, or progressive stenosis/regurgitation (due to prosthetic valve dehiscence). Intraprosthetic regurgitation occurring late after TAVI is suggestive of infective endocarditis, particularly when it is not associated with valve stenosis.13
Bioprosthesis Thrombosis
Bioprosthesis thrombosis has been reported in two different forms. First, as a symptomatic obstructive valve thrombosis, resulting in an increase in the transvalvular gradient and reduction in the effective orifice area measured by echocardiography, which is a rare event and reported in about 0.5% of TAVI patients.13,17 Second, as asymptomatic subclinical valve thrombosis causing thickening and reduced leaflet motion of bioprosthetic aortic valves detected by CT scan with normal transvalvular gradients at transthoracic ECG. This form is reported to be more frequent in patients treated percutaneously, ranging from 5% to 40% of TAVI patients.18 The incomplete expansion of a TAV’s stent as well as its metallic nature seem to be two of the main factors increasing the risk of subclinical thrombosis.13
Paravalvular Leak
Paravalvular leak is mainly caused by the incomplete sealing of the prosthesis to the aortic annulus. Although this is more frequently an early finding, the importance of paravalvular regurgitation may become clinically evident later as it contributes to leaflet degeneration. Treatment mainly consists in re-intervention or percutaneous paravalvular leakage closure. Next-generation TAVs have incorporated additional features with the aim to prevent paravalvular regurgitation, such as the possibility to recapture and reposition the device and the implementation of an external sealing skirt in the inflow portion of the valve.
Definitions and Diagnosis of Structural Valve Degeneration
SVD in bioprosthesis has been poorly defined for a long time. Traditionally, it is considered an acquired intrinsic bioprosthetic valve abnormality characterised by the deterioration of the leaflets or supporting structures. One of the causes of SVD is the mechanical stress and the abnormal flow at the surface of the leaflet, which leads to tissue disruption or thickening, collagen fibre disruption and tissue calcification, but the precise mechanism is not known. Risk factors often associated with bioprosthetic SVD are younger age, mitral valve position, end-stage renal disease, higher calcium-phosphorus product, hyperparathyroidism, hypertension and pregnancy.19–22 Other clinical valve abnormalities that do not cause deterioration of valve tissue, are not included in the definition of SVD. These include patient-prosthesis mismatch, device malposition, paravalvular regurgitation and abnormal frame expansion in the case of self-expanding transcatheter bioprostheses.10,13,22 Prosthetic valve thrombosis and infective endocarditis are not included in the definition of SVD, but these complications may subsequently lead to SVD, even if treated successfully.17,23,24
The lack of standard definitions for bioprosthesis dysfunction have affected the possibility of a proper comparison of durability studies. Most studies regarding surgical prosthesis have associated SVD with the need for reoperation, but did not provide any specific criteria to define SVD and/or the indication for reoperation. Furthermore, reoperation does not necessary imply presence of SVD as well as SVD does not always lead to reoperation, and older patients are often refused redo surgery because of their high or prohibitive surgical risk profile and their frailty.25 With the worldwide increase in TAVI procedures and the expansion of its indication to include younger, lower-risk patients, a clear definition of TAV durability and comparison with its surgical counterpart is needed.
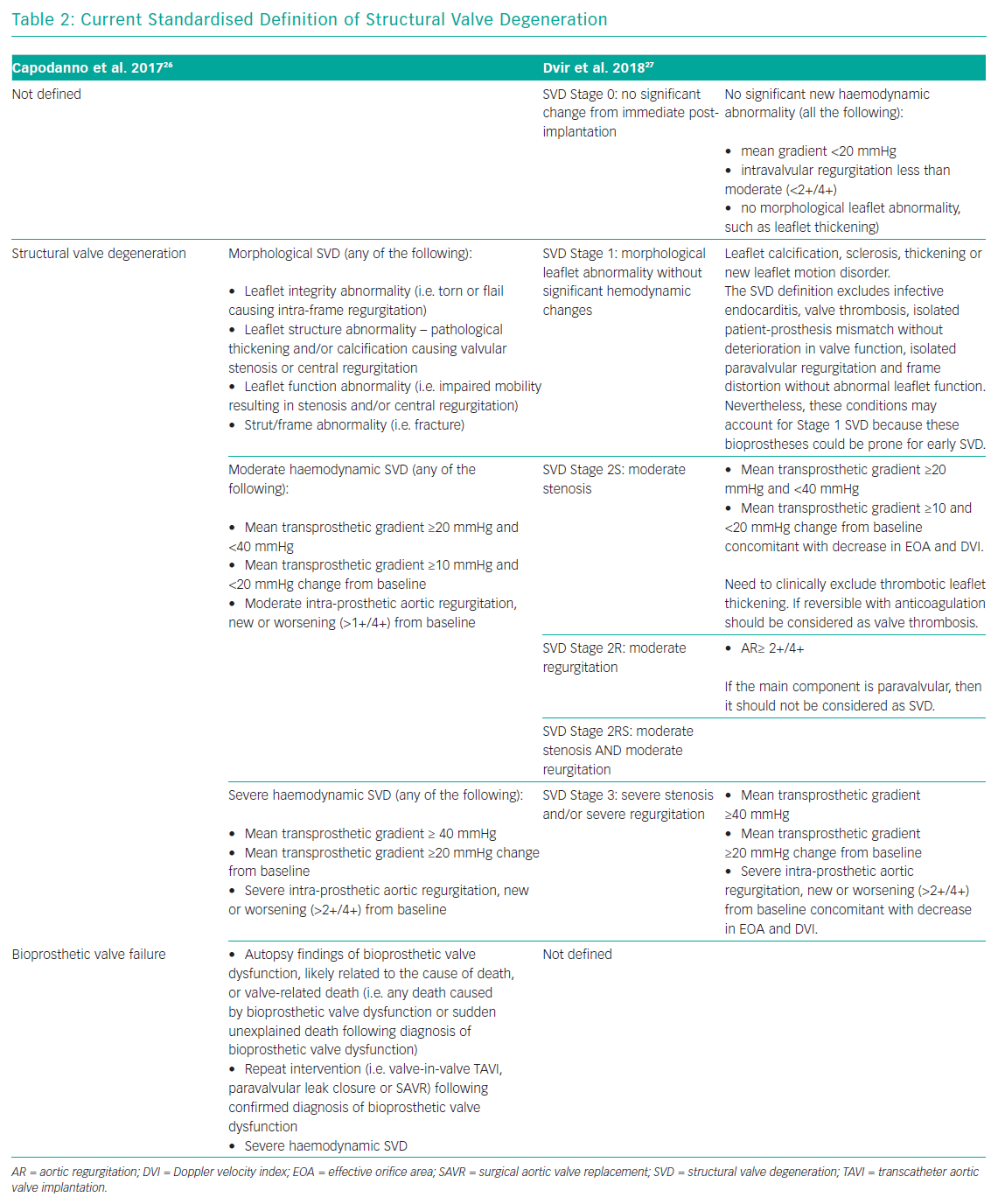
In 2017, a taskforce of the EAPCI, ESC and EACTS released new standardised definitions of SVD and a new patient-oriented clinical endpoint named bioprosthetic valve failure (BVF), with the aim of making possible future comparisons between studies on bioprostheses’ long-term durability.26 The taskforce characterised structural valve dysfunction as ‘haemodynamic SVD’ and/or ‘morphological SVD’. Haemodynamic SVD is defined as the presence of permanent changes in valve function assessed by echocardiography, even without evidence of morphological SVD. There are two different degrees of haemodynamic SVD. Moderate is defined by the presence of a mean gradient ≥20 mmHg and <40 mmHg and/or ≥10 mmHg and <20 mmHg change from gradient at baseline (valuated before discharge or within 30 days of valve implantation) and/or the onset of moderate, new or worsening (>1+/4+) of intra-prosthetic valve regurgitation; severe, with mean gradient ≥40 mmHg and/or ≥20 mmHg change from baseline and/or severe, new or worsening (>2+/4+) intra-prosthetic aortic regurgitation. Morphological SVD includes abnormalities in leaflet integrity, structure, function and strut/frame. The diagnosis is based on imaging or autopsy findings. BVF definition integrates severe haemodynamic SVD and its clinical consequences. It is important to stress that BVF could be a consequence of pathophysiological processes unrelated to SVD, such as thrombosis, endocarditis or non-structural valve dysfunction. BVF includes any of the following (Table 2):
- Bioprosthetic valve dysfunction at autopsy, very likely related to the cause of death, or ‘valve-related death’, defined as any death caused by bioprosthetic valve dysfunction in the absence of confirmatory autopsy.
- Aortic valve reintervention (i.e. valve-in-valve TAVI, paravalvular leak closure or SAVR).
- Severe hemodynamic SVD.
In 2018, the Valve In Valve International Data (VIVID) group proposed a definition of SVD which emphasises the progressive character of bioprosthetic valve degeneration, similar to that of the native valve, and provided recommendations for timing of clinical and imaging assessment at follow-up.27 The first stage (Stage 1) is defined as early morphological leaflet changes without haemodynamic sequelae, such as leaflet fluttering and leaflet thickening, and limited, delayed or asymmetrical leaflet opening or closure. Valves with definite morphological abnormalities are classified as Stage 1 SVD even if the abnormality is corrected, such as leaflet thickening relieved with anticoagulation, because these leaflets might still be prone to recurrent leaflet thickening or accelerated SVD, requiring more frequent follow-up. Stage 2 SVD refers to morphological abnormalities of valve leaflets associated with haemodynamic dysfunction. This stage is divided into Stage 2S and Stage 2R, depending on the kind of dysfunction, stenosis or regurgitation respectively, because the clinical implications and pace of deterioration are likely to differ between these two failure modes. Stage 2S can be determined by the mandatory presence of an increase of transvalvular gradient (≥10 mmHg) and a concomitant decrease in valvular area. In this way, the presence of abnormal leaflet morphology and deterioration in valve haemodynamic must coexist to define SVD. Furthermore, bioprosthetic valves with a combination of moderate stenosis and moderate regurgitation may be considered differently than those with isolated stenosis or regurgitation.28 VIVID definition categorises these mixed moderate stenosis/regurgitation valves at Stage 2RS. Patients in stage 2 could be considered for reintervention in case of symptoms’ onset. The most severe stage of SVD is the development of severe stenosis or regurgitation (stage 3). Reintervention is recommended when patients with stage 3 bioprosthesis SVD start to develop symptoms (Table 2).
Bioprostheses Durability Data
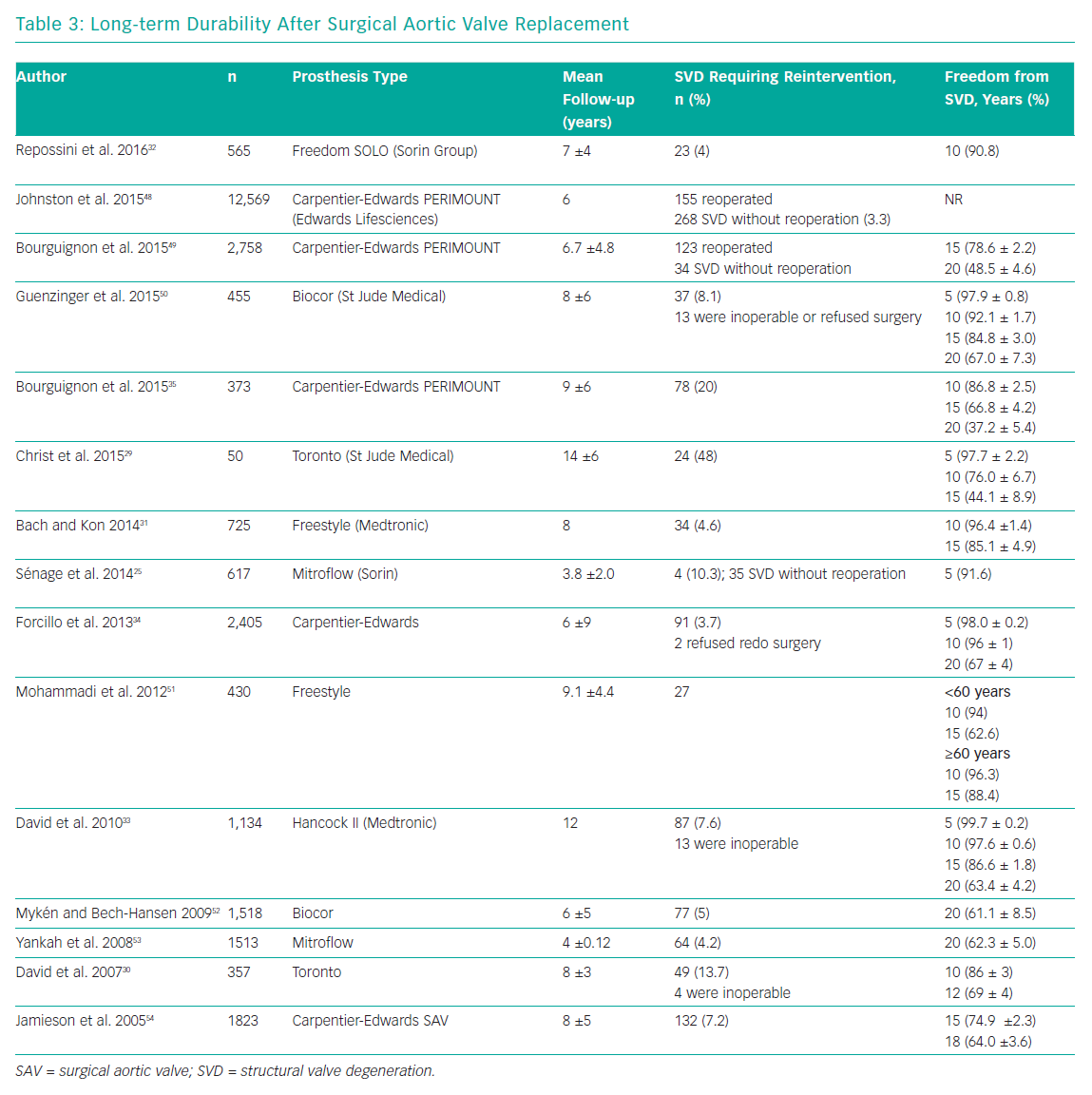
Long-term durability is one of the main limitations of surgical bioprostheses, compared with their mechanical counterparts. Several studies have reported encouraging data on valve performance during the first decade after valve implantation, with rates of freedom from SVD >85% at 10 years.29–33 Data on surgical bioprostheses durability at 20 years are limited. Two studies reported results about durability of the Carpentier-Edwards Perimount pericardial bioprostheses (Edwards Lifesciences) at 20 years. Forcillo et al. reported 67% rate of freedom from reoperation at 20 years, whereas Bourguignon et al. reported a rate of freedom from SVD of 48.5%.34,35 Unfortunately, the lack of standard definitions for valve dysfunction affects any comparison between different studies (Table 3).
It is well known that transcatheter aortic valves can degenerate in a manner similar to surgical bioprostheses, but durability of TAVs could be shorter than their surgical counterpart because of the possible trauma that can occur during initial valve preparation and compression, balloon dilatation or as a suboptimal leaflet coaptation, leaflet folding or leaflet-frame contact due to asymmetrical frame expansion.14
Several limitations prevent robust evaluation of TAV’s durability. Firstly, TAVI is a relatively young technology as its wider adoption started when it gained its CE mark in 2007 and US Food and Drug Administration approval in 2011. This means there can be little data on valve durability analysis beyond 10 years. Secondly, current data on long-term outcomes over 5 years refers to the first-generation TAVs, which were implanted by relatively inexperienced operators and and suffered from higher valve malpositioning rates and sizing problems. Finally, the major limitation of long-term durability evaluation is the older age of TAVI population, which is affected by multiple comorbidities and usually have high-risk profiles conditioning a limited life expectancy and therefore a paucity of patients (usually <50% of the initial population) available at long-term follow-up.
In the past few years the results of several TAVI studies with more than 5 years of follow-up data have been published. The Placement of AoRTic TraNscathetER Valve Trial (PARTNER-1) trial showed no evidence of SVD at 5-year follow-up.8,36 Moreover, the PARTNER-1A substudy demonstrated similar echocardiographic valve performance of TAVs and SAVs, with a mean transvalvular gradient of 10.7 and 10.6 mmHg, and an aortic valve area of 1.6 and 1.5cm2, respectively.8,37 This evaluation confirmed the satisfactory haemodynamic profile of transcatheter aortic valves up to 5 years post-implantation, even if moderate or severe aortic regurgitation caused by paravalvular regurgitation – which is not included in the SVD definition – was shown to be more common in the TAVI group. Toggweiler et al. showed a 3.4% incidence of SVD at a 5-year follow-up in a cohort of 88 patients who had received a balloon-expandable SAPIEN TAV (Edwards Lifesciences).6 Moderate aortic regurgitation, stenosis, or a combination of the two occurred in one patient each, and no patients required reintervention. Our group reported a 5-year experience with the CoreValve system (Medtronic) and found five cases (1.4%) of SVD, with two patients requiring reintervention (valve-in-valve) because symptoms developed 4 and 4.6 years after TAVI.7 Furthermore, 10 patients (2.8%) showed late mild stenosis, with a mean transaortic gradient ranging from 20 to 40 mmHg.7
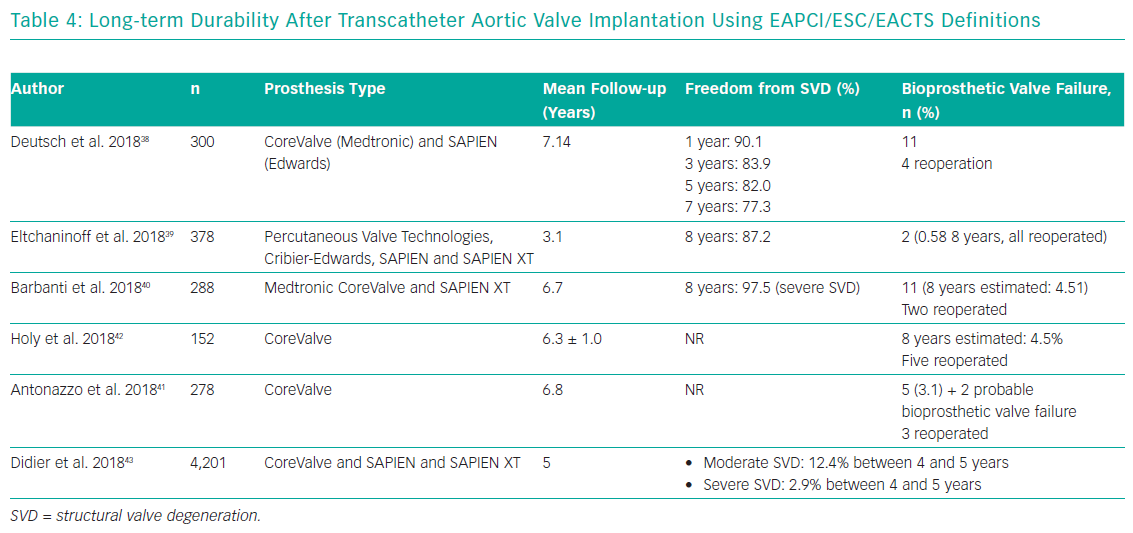
After the introduction of EAPCI/ESC/EACTS standardised criteria of SVD in 2017, an increasing number of studies have been reporting outcomes after TAVI with either the SAPIEN (Edwards Lifesciences) or CoreValve TAVs up to 7 and 8 years (Table 4).38–41 Three single-centre studies demonstrated stable trans-prosthetic gradients over time and a rate of severe TAV dysfunction of 2.4%, 3.2% and 3.6%.39–41 Holy et al. analysed long-term outcomes of 152 consecutive patients who had undergone TAVI with the self-expanding CoreValve between 2007 and 2011.42 Echocardiographic follow-up was achieved at 6.3 ± 1.0 years (5.0–8.9 years) and was 88% complete (60 of the 68 participants who survived beyond 5 years). No case of SVD was reported and five patients (3.3%) had undergone redo TAVI or surgery due to paravalvular leakage. An analysis by Deutsch et al. showed an overall crude cumulative incidence of SVD of 14.9% at 7 years after TAVI (CoreValve 11.8% versus SAPIEN 22.6%; p=0.01).38
Reports from national registries confirm low rates of TAV dysfunction at long-term follow-up. Data from the French Aortic National CoreValve and Edwards (FRANCE-2) registry showed an incidence of severe and moderate/severe SVD of 2.5% and 13.3%, respectively, in surviving patients at 5 years from the procedure, while Blackman et al. reported an incidence of severe and moderate SVD of 0.4% and 8.7% respectively up to 10 years in the UK TAVI Registry.43,44 Finally, a recent analysis from the Nordic Aortic Valve Intervention (NOTION) trial reported lower rates of moderate-to-severe SVD after TAVI compared with surgery (24% versus 4.8% for TAVI and SAVR respectively), whereas there was no difference in terms of BVF (6.7% versus 7.5% for TAVI and SAVR respectively).45
Structural TAV Dysfunction Management
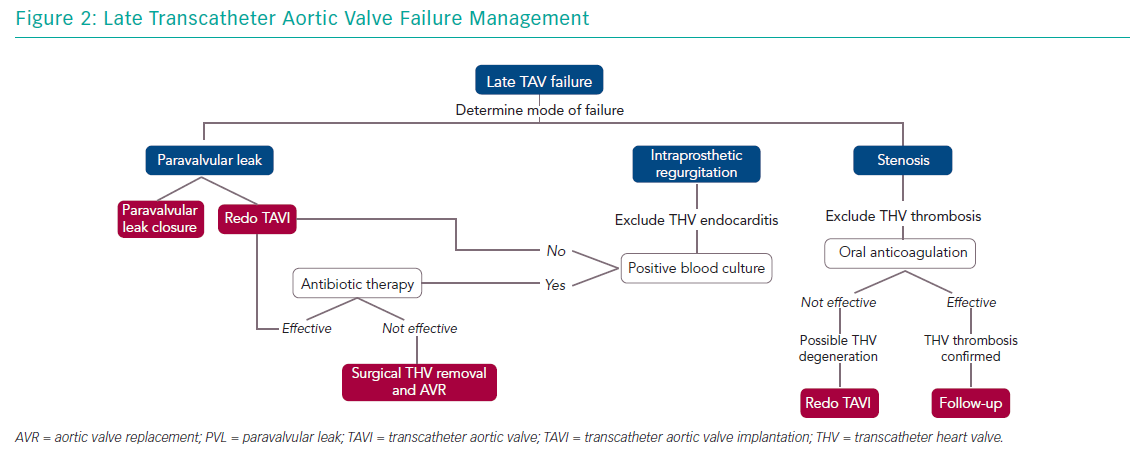
Management of older people with TAV failure is similar to that of surgical bioprosthesis dysfunction. Any decision about the appropriate approach should be guided by a careful clinical assessment and surgical risk evaluation by a heart team. Management options include medical therapy and reintervention with TAVI or SAVR. Understanding the causal mechanism of TAV degeneration is essential as treatment varies (Figure 2).
Intraprosthetic regurgitation occurring late after TAVI suggests infective endocarditis, particularly when not associated with stenosis.13 In this case, targeted antibiotic therapy represents the first line of treatment. In patients presenting with severe aortic regurgitation, transcatheter heart valve retrieval and SAVR should be considered. Redo TAVI is an alternative in those patients in which infection has been controlled and blood cultures are negative.14
In patients with suspected bioprosthesis thrombosis, oral anticoagulation is the treatment of choice both in patients with clear evidence of thrombosis and in those with high transvalvular gradient with evidence of reduced leaflet motion. This therapeutic approach is often sufficient to restore transvalvular gradient and leaflet mobility.17 Vitamin K antagonists are the most used anticoagulants but novel oral anticoagulation therapy has also been shown to be effective.46 In obstructive TAV thrombosis, anticoagulation has been shown to normalise transvalvular gradients in 2–3 months and patients should have lifelong follow up with interval imaging.17 SAVR should be considered for patients with worsening valve function despite oral anticoagulation therapy. The observation of valve thrombosis has raised the question of treatment necessity with oral anticoagulation for a certain period after TAVI to prevent leaflet thrombosis, but the debate is still ongoing.
Redo TAVI is another option in the management of TAV failure. This strategy has been demonstrated to be safe and it is associated with favourable clinical and echocardiographic outcomes.47 When a balloon-expandable TAV fails, implantation of a second same-sized balloon-expandable TAV is the most commonly used approach.47 On the other hand, a supra-annular TAV may be preferable in cases of small native annulus due to the possibility of a high residual transvalvular gradient. Redo TAVI strategy has several advantages:
- It is safe, with lower risk of periprocedural complications compared with redo SAVR, which is technically challenging and carries a higher risk of mortality and morbidity than the first valve procedure.12
- The presence of the first device represents a fluoroscopic marker for the landing zone, indicating second TAV sizing and facilitating deployment.
Despite its advantages, there are two main concerns associated with redo TAVI: the lack of data on the long-term performance of valve-in-valve implantation; and the access to coronary arteries, especially in the case of a second self-expanding CoreValve device implantation when a double layer of close cell metallic frame would face the coronary ostia.
When the mechanism of TAV failure is paravalvular regurgitation, there are two possible scenarios: the paravalvular regurgitation is mainly caused by valve implantation that is too high or too low; or it is secondary to incomplete frame expansion or suboptimal sealing because of severe annular calcification. In this latter case, balloon post-dilatation could represent an alternative, although it may cause annular injury when large-diameter balloons are used. However, for either scenario, potential treatments could be represented by a redo TAVI procedure using any TAV type in the proper position (in the case of high or low implant), or a second TAV with higher radial force – SAPIEN or Lotus (Boston Scientific) – if greater expansion and sealing are required. In addition, paravalvular regurgitation closure with dedicated devices (plugs) has been demonstrated to be a feasible and safe alternative.
Conclusion
With the expansion of TAVI indication to younger, lower-risk patients, data on long-term TAV durability are essential. The first studies reporting on SVD up to eight years after TAVI showed very low rates of TAV degeneration, comparing favourably with its surgical counterpart. The release of standardised definitions of SVD represents a fundamental step in allowing data comparison from different centres, with the aim of obtaining a better insight into its real incidence. Evidence suggests that redo TAVI seems to be a feasible and safer alternative to surgery for treatment of bioprostheses failure.