Transcatheter aortic valve replacement (TAVR) has become the default treatment option for high-risk patients with aortic stenosis (AS) and, based on heart team discussion, an alternative to surgical aortic valve replacement (SAVR) in intermediate-risk patients.
TAVR has led to a paradigm shift in the basic therapeutic principle to treat AS: calcifications, in some patients quite excessive, are being squeezed aside instead of carefully surgically resected before valve placement. Due to these differences, the incidence of cerebrovascular events has been one of the main concerns associated with TAVR. In the Placement of AoRTic TraNscathetER Valve (PARTNER I) trial comparing TAVR and SAVR in a high-risk but operable patient cohort, stroke rates were higher after TAVR.1,2 Further studies showed that perioperative stroke leads to a five-fold increased risk of mortality after TAVR. 2,3 Any technique to minimise the incidence and the risks for stroke during TAVR is therefore of the utmost importance.
The present review summarises the aetiologies and potential strategies for managing stroke during TAVR.
Stroke Definition
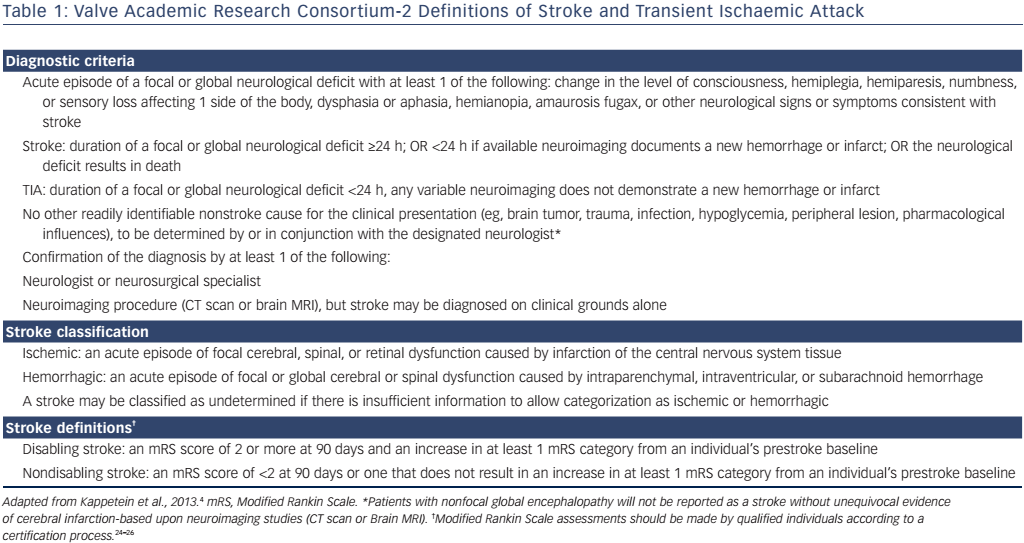
The current Valve Academic Research Consortium (VARC)-2 consensus defines stroke as: “an acute episode of a focal or global neurological deficit” (see Table 1).4 Stroke is classified as ‘undetermined’ if there is no further information available as to whether it is ischaemic or haemorrhagic. Time-wise a neurological event (NE) is called ‘stroke’ if it lasts longer than 24 hours or less than 24 hours with new equivalents in neuroimaging or if the neurological deficit results in death. A NE is otherwise classified as transient ischaemic attack.
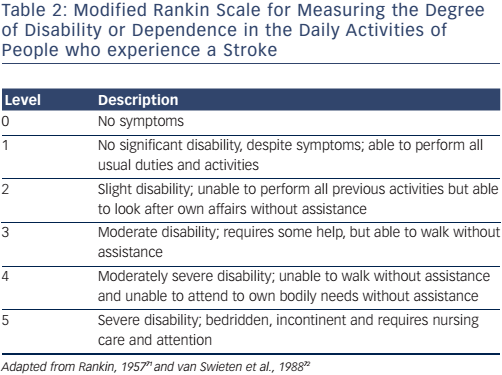
The VARC-2 consensus recommends the utilisation of the modified Rankin Scale (mRS) to measure disability after stroke (see Table 2). This is important, as stroke may be diagnosed on clinical findings alone without confirmation by specific imaging.
It is recommended the terms ‘disabling’ and ‘non-disabling’ are used for stroke classification instead of ‘major’ and ‘minor’, respectively. ‘Disabling’ is defined as an mRS score of >2 points or an increase in >1 mRS category from pre-stroke baseline; ‘non-disabling’ as <2 mRS score points or without an increase in >1 category in mRS from baseline. The mRS should be assessed by a professional neurologist experienced in clinical trials 90 days after stroke onset.5 The Neurologic Academic Research Consortium recently proposed standardized neurological endpoints for cardiocascular clinical trials and aims to differentiate between clinically meaningful and incidential findings. The recommended classification includes overt and covert central nervous system (CNS) injuries and neurological dysfunctions without CNS injury.
Incidence
Stroke is a quite rare but major complication after SAVR and is known to occur in 1.3–1.7 % of patients.6,7 In TAVR, however, initial stroke rates are higher. The PARTNER trial reported a NE rate that was twice as high in TAVR patients compared to SAVR in the high-risk cohort (cohort A), with 5.5 % versus 2.4 % at 30 days (p=0.04) and 8.3 % versus 4.3 % at 1 year (p=0.04).8 However, recent publications from large registries show lower stroke rates. The Transcatheter Valve Therapy Registry included 7,710 patients who received TAVR using both transvascular (TV) and transapical (TA) approaches between 2011 and 2013.9 An = overall stroke rate of 2.0 % (95 % CI [1.7–2.4]) was reported during hospital stay and 2.8 % (95 % CI [2.3–3.5]) at 30 days. The European SAPIEN Aortic Bioprosthesis European Outcome (SOURCE) registry showed a similar 30-day incidence of stroke of 2.5 %.10 The German Aortic Valve Registry (GARY) is the largest registry including both SAVR and TAVR patients. It has reported an overall in-hospital stroke rate of 2.3 %. Although there was a trend towards higher stroke rates in TA-TAVR, there were no significant differences between SAVR, TV-TAVR and TA-TAVR.11 Other reports suggested a higher incidence of NEs (up to 6.0 %) using the TV approach.12,13 As no larger study was able confirm these data, however, whether the access impacts stroke rates remains controversial.3,10,14,15
When comparing PARTNER Ib with the PARTNER II trial, a significant decline in NEs, such as strokes, can be seen over time. The PARTNER Ib trial reported a stroke rate of 5.0 % at 30 days and 7.8 % at 1 year in 2008 compared to 3.1 % at 30 days and 5.2 % at 1 year in the PARTNER II trial in 2013.16 These findings are supported by a metaanalysis comparing early versus late data from high-volume centres, showing a decline in strokes from 4.9 % to 3.4 % at 30 days.17 Thus, the observed decrease in stroke rates over time is most likely a result of experience gained in patient selection and implantation, improved and smaller TAVR devices, and lower-risk patients. Any risk of stroke, which is fortunately down to 2 to 3 %, still is of clinical concern for patients.
Registries appear to have lower stroke rates than randomised trials when reported percentages are compared. One possible explanation for the this could be the more elaborate stroke diagnosis, including expert neurologist consultation, in large prospective trials. Registry results, in comparison, are usually based on self-reporting only.
Timeline and Aetiology
Stroke after TAVR occurs in three distinct phases:
- early: in high-risk patients, this is a directly procedure-related phase, with up to 50 % of all NEs happening in the first 24 hours after TAVR;18–22
- delayed: this occurs in patients with an elevated risk interval between days 2 and 30; and
- late: stroke occurs in those with a late hazard interval and is mostly related to patient- and disease-related factors.
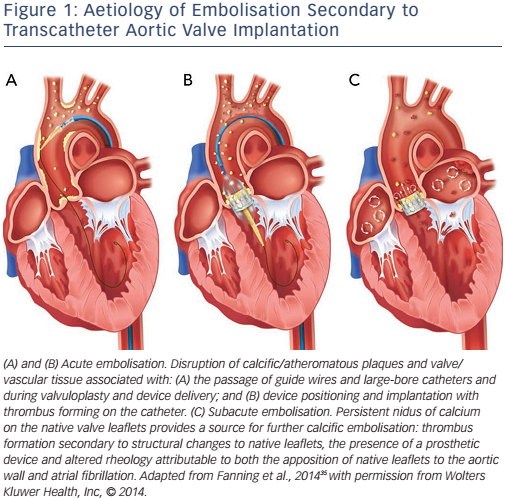
These phases are caused by the different aetiologies of stroke after TAVR, see Figure 1.
Phase One: Early stroke
A number of brain imaging studies using diffusion weight magnetic resonance imaging (DW-MRI) technology in patients before and during the first days after TAVR have investigated the early onset of stroke.23–27 All these trials showed similar results. DW-MRI detected new lesions in the majority (up to 84 %) of patients after TAVR. Only a small proportion of these patients (up to 6 %), however, presented with new and apparent clinical symptoms of stroke. Fairbairn et al. identified a correlation between clinical strokes and the number and volume of new lesions, but their findings were based on a small study (n=31).26 Rodés-Cabau et al. found a trend towards a lower occurrence of new lesions after TA (66 %) versus TV (71 %) TAVR in a small cohort (n=60) but the difference was not statistically significant (p=0.78).25
Due to the pattern of the multiple and dispersed lesions in both hemispheres, it has been suggested that embolic events may cause these lesions.23–27 Histopathological investigations have confirmed this embolic theory. Van Mieghem et al. investigated particles saved by the Montage™ Dual Filter System (Claret Medical, Inc) dual filterbased embolic protection device (EPD) during TAVR.28 Debris was found in 75 % of all cases and was specified as fibrin, calcium and connective tissue most likely originating from the calcified native aortic valve and the aortic wall. Furthermore, equipment-related debris in the form of catheter shavings were found.28 Support for the embolic theory has been provided by the results of transcranial Doppler studies quantifying high-intensity transient signals and microembolic signals during TAVR.29 Although high-intensity transient signals were seen in all procedural TAVR steps, there were peaks during valve positioning and implantation, leading to the conclusion that the main source of microembolisation is in the area of the calcified stenotic native aortic valve.30–32
Manipulation of the vessels during valve delivery and the procedure itself can cause thrombus and potential emboli formation. Endothelial integrity damage uncovers tissue factor and thrombin, especially in atherosclerotic tissue such as calcified valve leaflets. Tissue factor, as the main initiator of coagulation, leads to the activation of plasmatic coagulation cascades and cellular aggregation, resulting in inflammation and thrombogenicity.21,33,34 In addition, wires, catheters, balloons and delivery systems used during the procedure are known to be prothrombotic and are potential sources of air emboli, leading to an increased risk of stroke.35
The previously-mentioned factors are the main causes of stroke during the early procedure-related high-risk phase of TAVR. In addition to strokes with ischaemic causes, 5.0 % are haemorrhagic.36,37
The highly reduced blood flow in the watershed areas of the brain vessels during balloon aortic valvuloplasty (BAV) and rapid pacing results in decreased washout of the embolised particles, and thus to an increase in ischaemic effects from embolised debris.38,22
Phase Two: Delayed Stroke
After the initial TAVR procedure is done, thrombogenicity resulting in thromboembolism extends its role. New-onset atrial fibrillation (NOAF) is a known post-procedural complication after heart surgery correlating with left atrial size, volume management, inflammation, medication and extracorporal circulation.39 By VARC-2 criteria, NOAF is defined as any episode of atrial fibrillation (AF) lasting long enough to be recorded on a 12-channel electrocardiogram or at least 30 seconds on a rhythm stripe without prior history of AF.4 In TAVR, NOAF is described in 7.2–32 % of patients and is an independent predictor of delayed stroke. 40,41 Nuis et al. showed a 4.4-fold increase in the risk of stroke in the presence of NOAF compared to patients without NOAF.36 Nombela-Franco et al. reported an odds ratio of 2.76.37 Amat-Santos et al. described an accentuated stroke rate of up to 40 % in patients with NOAF without anticoagulation compared to 2.9 % in patients with immediate anticoagulation therapy (p=0.008).40 In all cases of delayed stroke in TAVR, the occurrence of NOAF could be evaluated retrospectively, with an onset between days 1 and 30 after TAVR.35–37,40 Another reason for the increased thromboembolic risk is the comparatively long period of time until the artificial nitinol surfaces of TAVR valves are endothelialised.42
Phase Three: Late Stroke
With an indistinct transition from NOAF to chronic AF, the late hazard interval begins to emerge. These delayed strokes are more likely patient-related. Commonly reported and pre-existing diseases in patients with aortic stenosis are arterial hypertension, metabolic disorders like diabetes mellitus, dyslipidaemia. Other common factors include obesity, female sex, older age and nicotine addiction. All of these factors result in a protruded risk of atherosclerosis, resulting in a higher risk for cerebrovascular disease.43,44 In comparison, the annual stroke incidence for a healthy octogenarian is about 1.0–2.3 % and is thus comparable to stroke incidence after 30 days.45
Managing Stroke
Preprocedural Strategies
Fairbairn et al. showed that the severity of aortic arch atheroma, catheterisation time and age are risk factors for stroke after TAVR.26 Miller et al. also found that a smaller aortic valve area index, which contributes to a higher degree of valve calcification, may be an prediction factor for stroke.2 Furthermore, risk factors for AF, such as larger left atrial size, should be evaluated before the procedure as NOAF is associated with an increased risk of stroke after TAVR.37,36,40,46
Risk stratification and a multidisciplinary heart team are essential for preprocedural stroke management in order to select the most appropriate approach for the treatment of patients with symptomatic AS. Conventional surgery and transcatheter options should be discussed in relation to the current guidelines, as well as anaesthetics, anticoagulation therapy and the use of EPDs, depending on individual patient risk and the patient’s current state of health.47
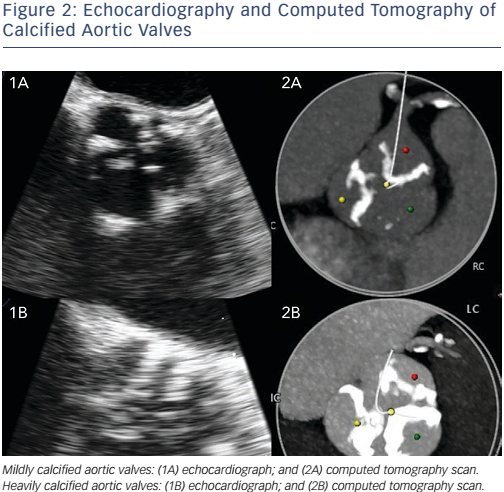
Imaging of the aortic annulus and access site by computed tomography and echocardiography are crucial in preprocedural planning of the approach and determining the correct sizing and best valve type to minimise the probability of valve misfit, under-expansion and malposition (see Figure 2).21,48,49
Procedural Strategies
Embolisation is the predominant cause of new NEs in the early postprocedural high-risk phase. The essential preventive strategy for reducing embolisation risk is to minimise manipulation in the area of the native aortic valve. Less trauma may also contribute to the size and rigidity of the catheters and devices used.49 In the PARTNER trial, with the early SAPIEN™ (Edwards Lifesciences Inc.) valve the catheters used were 22–24 F sized. The current generation of TAVR systems are 18 F, such as the CoreValve® Evolut™ R (Medtronic Inc.) or the Edwards SAPIEN 3™, are 18 F.17,47
In a transcranial Doppler study it was found that valve positioning and valve implantation are the most crucial triggers of intraprocedural embolisation during TAVR.31 The aortic arch seems to play only a minor role in embolisation, which may contribute to a comparable risk of stroke in TA and TV TAVR. Kahlert et al. showed a higher risk of embolisation using the self-expandable CoreValve® compared to the balloon-expandable SAPIEN™. This might be related to the longer stent combined with the slow and stepwise release of the CoreValve® resulting in prolonged and therefore more severe scraping inside the native valve and vessels.31 Smooth passage of a catheter through the aortic arch, depending on device length and flexibility, may be additional factors that minimise embolisation risk. Thus, it might be expected that the rapid implantation of the SAPIEN™ would be associated with a reduced risk of embolisation. Studies have, however, shown similar stroke rates for both TAVR systems.17 Pre-ballooning via BAV seems to a have a relatively low risk of embolisation; however, data show that post-deployment BAV results in fewer high-intensity transient signals than pre-ballooning.31 Grube et al. showed a lower stroke incidence in a trial using the CoreValve® without BAV (5.0 %) compared to pre-dilatation (11.9 %).50 Skipping the utilisation of rapid pacing while performing CoreValve® may be of additional benefit regarding washout phenomena in the watershed areas of the brain.22,38,50 Balloon-expandable valve insertion techniques without previous BAV are now routine in clinical practice.51–54
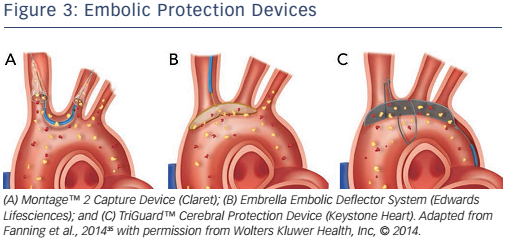
Another periprocedural strategy for managing stroke and embolism is the use of EPDs. EPDs are designed to avoid cerebral embolism by capturing or deflecting debris from the cerebral circulation.55
The Sentinel™ Cerebral Protection Device (Claret Medical Inc.) is a filtering device placed in the brachiocephalic trunk and left common carotid artery, see Figure 3A. Its ability to capture debris has been shown in histopathological studies.28,56 Although debris has been found in in up to 75 % of patients who have undergone TAVR with the use of the Sentinel™, no degree of functional brain protection was able to be evaluated from these findings. The larger single-centre randomised blinded Claret Embolic Protection and TAVI (CLEAN TAVI) trial found that the number and volume of new lesions detected in DW-MRI were significantly lower in TAVR when using EPDs.57
The Embrella Embolic Deflector System ([EEDS], Edwards Lifesciences Inc.) is a microporous screen that deflects along the great curve of the aortic arch, see Figure 3B. It got CE mark approval in 2010 and was investigated by the Prospective Outcome study in patients undergoing TAVI to Examine Cerebral Ischemia and Bleeding Complications (PROTAVI-C) trial.58 The EEDS was proved to be feasible, safe and easy to deploy, but the PROTAVI-C trial showed an even higher rate of microemboli in the EEDS group compared to the group without EEDS in TAVR, as confirmed by transcranial Doppler. DW-MRI showed new lesions in all patients, however the lesions were potentially smaller in the EEDS group. No recommendation for systematic use was given after the trial.
The TriGuard™ Cerebral Protection Device (Keystone Heart), see Figure 3C, received CE mark approval in 2013 and it consists of a nitinol mesh designed to deflect debris. The multicentre Prospective, Randomized Evaluation of the TriGuard™ HDH Embolic Deflection Device During TAVI (DEFLECT III) trial59 detected fewer new neurological defects using the National Institutes of Health Stroke Scale at discharge (15.4 % versus 3.1 %; p=0.16) and demonstrated a >2-fold increase in recovery of normal cognitive function at 30 days in those randomised to TriGuard™ protection compared to those with no protection. There was also a reduction in lesion volume and number on DW-MRI. Although the results are promising, stroke rates were not significantly lower in the TAVR group protected by TriGuard™ (4.3 % versus 5.1 %; p=0.87)
EPDs seem to be a promising approach to decreasing the incidence of stroke during TAVR. A reduction in the frequency of ischaemic lesions originating from the calcified native aortic valve has been seen; however, routine clinical use has not yet been established due to preliminary data from current trials. Further investigation is needed to assess the effects of EPDs on NEs.57 The benefits of EPDs, it should be noted, are directly limited to the timeframe of the procedure itself.
Antithrombotic management plays a significant role in reducing stroke risk during and after TAVR, but little is known about optimal antiplatelet and anticoagulation therapy. The joint American College of Cardiology Foundation/American Association for Thoracic Surgery/Society for Cardiovascular Angiography and Intervention/Society of Thoracic Surgeons guideline recommends intraprocedural use of heparin with an activated clotting time of >300 seconds.47 In the PARTNER trial, a loading dose of 5,000 IU of heparin and an activated clotting time of >250 seconds was recommended.1,8
The use of antiplatelet medication after TAVR due to the stentmediated risk of thrombosis has not been determinately defined. PARTNER recommended the use of dual antiplatelet therapy (DAPT) with 75–100 mg aspirin on daily basis plus 75 mg clopidogrel for 6 months after TAVR, with a loading dose of 300 mg directly after the TAVR procedure.60,61 These recommendations are not based on larger randomised multicentre trials and are not specifically defined in guidelines. The use of DAPT with clopidogrel particularly has been questioned in TAVR patients due to its excessive bleeding risk and an unclear beneficial effect.62,63 Durand et al. showed a significantly increased risk for major bleeding in the DAPT group without a lower risk of ischaemic events compared to monoplatelet therapy using aspirin.62 Controversially, in a more recent study Gleason et al. showed that DAPT was associated with a decline in early stroke rate in TAVR.64 The study by Linke et al. supports treatment with antiplatelet substances due to the fact that the nitinol stent surface of the TAVR is known to be thrombogenic and coverage takes up to 1 year.42 Other long-term data indicate that neointimal tissue growth and coverage of bioprosthetic valves with endothelial cells occurs about 3 months after implantation, which is about the time when the risk of stroke after TAVR adjusts to a comparable population risk.65 DAPT for 3–6 months is probably the most commonly applied approach in clinical centres at present after TAVR.
These results require further investigation in larger randomised multicentre trials and the production of defined guidelines.
Post-procedural Strategies
After discharge, thromboembolic risk is still imminent. Individual antithrombotic and antiplatelet management may be the key to a safe and event-free long-term outcome after TAVR. NOAF is a predominant risk factor for thromboembolism after TAVR and occurs in up to one-third of all patients.35–37,40 Despite this, no defined guidelines are proposed for antithrombotic management after short episodes of NOAF following TAVR.40,66 Nuis et al. and Amat-Santos et al. gave a preoperative median congestive heart failure, hypertension, age, diabetes, prior stroke or transient ischaemic attack or thromboembolism (CHADS2) score of 3 (interquartile range 2–4), which supports the theory that TAVR patients are at high risk of thromboembolism when NOAF occurs and anticoagulation therapy should be implemented immediately after NOAF onset.36,40 In the case of AF, anticoagulation with a vitamin k antagonist such as phenprocoumon or warfarin is recommended, combined with one antiplatelet substance such as aspirin or clopidogrel. The target of anticoagulation is an international normalised ratio of between 2 and 3.66–68
NOAF is a risk factor for stroke, especially between days 1 and 30. Later on other factors and diseases are responsible for an increased risk of stroke. These factors and diseases should be treated to avoid progression of arteriosclerosis as a distinct part of cerebrovascular events.21
Conclusion
It is essential to identify patients who are at high risk of stroke during procedural planning. Preventive strategies such as anticoagulation and antiplatelet therapy, less traumatic devices, the avoidance of extensive manipulation while performing TAVR and the use of EPDs in special cases are key to significantly reducing NEs.69 Experience in imaging, anaesthesia, valve choice and medical treatment is required; a heart team approach is thus needed to ensure the best possible therapy and clinical outcome for each patient.47 Prediction algorithms focusing on TAVR-specific stroke risks are in development and might further ease decision-making.70 Patient characteristics and technical possibilities will change the risk of stroke in the future.49 Larger multicentre randomised trials are needed to gain further insight into the specific mechanisms and possible prevention of stroke after TAVR.