Over the past decade, transcatheter aortic valve implantation (TAVI) has become the treatment of choice for elderly patients with symptomatic severe aortic stenosis at high risk for death or complications with conventional surgical aortic valve replacement.1–4 Recent studies and clinical practice confirmed TAVI feasibility in patients with a lower risk profile who are typically younger and have a longer life expectancy.5 In multiple randomised trials comparing TAVI with surgical aortic valve replacement, clinical stroke rate was similar for the two strategies.6 Still, TAVI comes with a substantial ~ 5 % post-procedural cerebrovascular event rate, which predominantly occurs in the first 48 hours after TAVI.7–9 This suggests a causative role between the procedure and cerebrovascular events and thus could indicate the need for preventive procedural measures, such as embolic protection devices (EPDs). Moreover, brain MRI studies have consistently shown signs of brain injury after TAVI.6 These new brain lesions may be clinically silent yet not trivial, as they are linked to neurocognitive decline and frank dementia.10 All these findings plead for strategies to minimise per-procedural embolisation to the brain. Various EPDs have been designed to prevent emboli from entering the cerebral circulation and should be inserted before navigating through the aortic arch.11 Embolic deflectors are deployed in the aortic arch and reroute debris away from the brain into the descending aorta; current filter-based systems are deployed in the brachiocephalic trunk and left common carotid artery to capture and remove debris travelling to brain. In this overview we discuss the safety and efficacy of EPDs and put clinical data in perspective of histopathological findings of the material that embolises to the brain during TAVI.
Clinical EPD Data
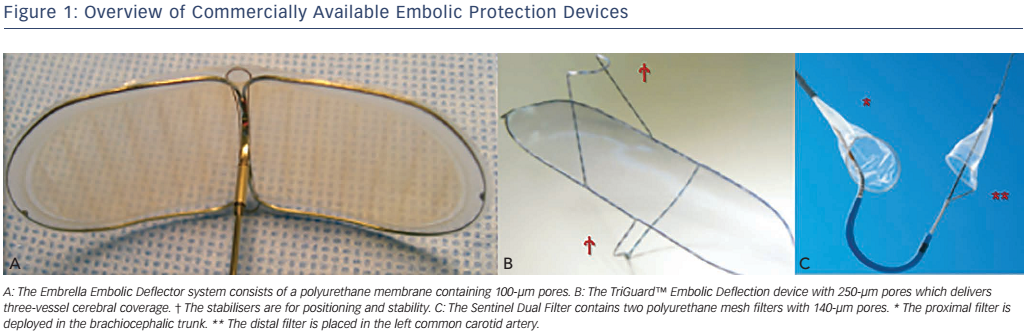
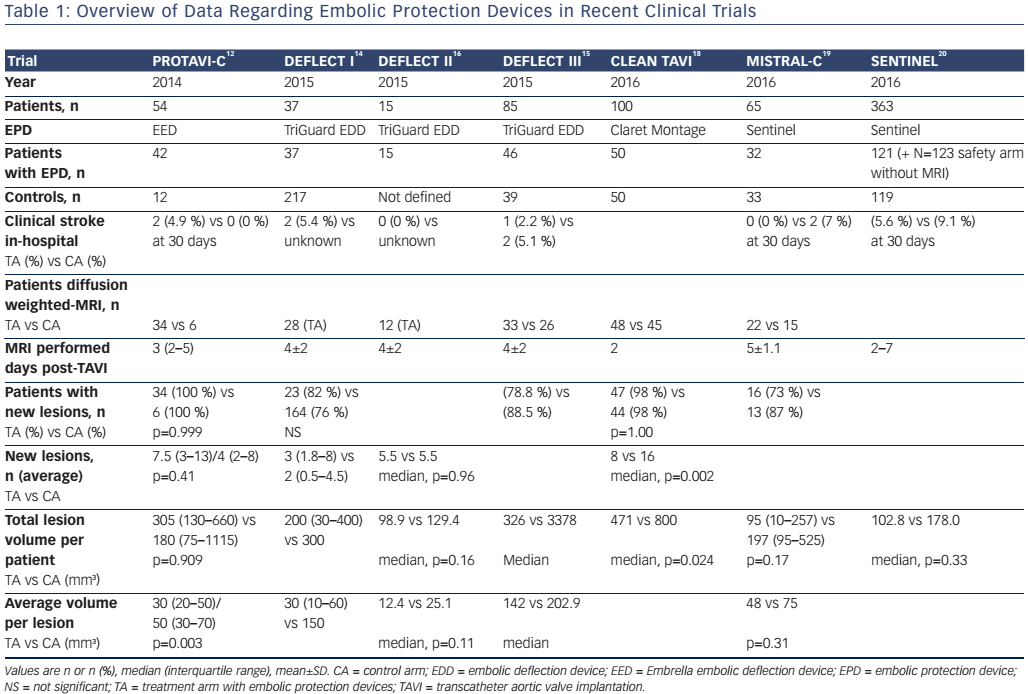
The Embrella Embolic Deflector (Edwards Lifesciences Ltd) and the TriGuard (Keystone Heart Ltd) are deflecting devices that are deployed in the aortic arch to partially or completely cover the ostia of the brachiocephalic trunk and the left carotid and subclavian arteries (see Figure 1).
The Prospective Randomised Outcome Study in Patients Undergoing TAVI to Examine Cerebral Ischemia and Bleeding Complications (PROTAVI-C) trial was a pilot study of the Embrella (see Table 1).12 Use of Embrella resulted in an overall larger new brain lesion burden according MRI, with no effects on neurological events or neurocognitive function. Furthermore, a transcranial Doppler analysis suggested more high-intensity signals as surrogate for embolisation activity during the introduction and deployment of the device.13
Safety and feasibility of the TriGuard was evaluated in the SMT Embolic Deflection CE Mark Trial DEFLECT I study.14 Complete cerebral vessel coverage was obtained in 80 % of cases, although device instability was reported in approximately one-third of the cases. TriGuard use resulted in numerically lower total lesion volume detected by MRI compared to historical data of TAVI patients without protection. DEFLECT-III was a randomised controlled trial using a second generation TriGuard (with a smaller pore size of 130 μm compared to 250 μm on the first generation).15 Patients were randomised to TriGuard versus no protection and underwent MRI 4 days post TAVI. Complete cerebral vessel coverage was obtained in 89 % of cases. New neurological deficit (i.e. worse according to the National Institutes of Health Stroke Scale) appeared in 15 % of unprotected patients but only 3 % of TriGuard-protected patients. Neurocognitive decline at discharge and 30-day follow-up was more common in unprotected patients.15 Total brain lesion volume according to diffusion-weighted (DW)-MRI was lower in TriGuard-protected patients, particularly in patients undergoing TAVI with the SAPIEN 3 valve. Interestingly, TAVI using CoreValve (Medtronic) showed more device interference and less stability than SAPIEN 3 TAVI.16 The Cerebral Protection to Reduce Cerebral Emboli Lesions After Transcatheter Aortic Valve Implantration (REFLECT) study is currently underway and should provide further insights into Triguard. It randomises TAVI patients 2:1 into TAVI with TriGuard protection versus no protection. The primary endpoint is total volume of cerebral ischemic lesions on DW-MRI 2 to 5 days post procedure.
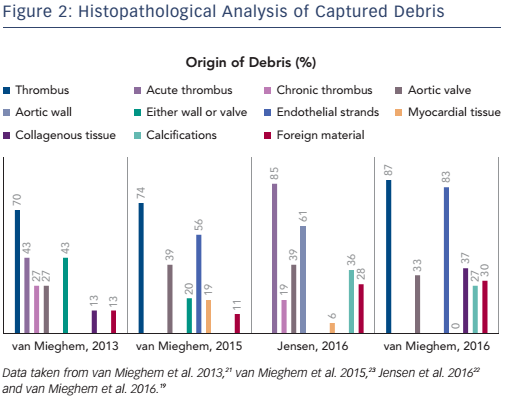
The Sentinel dual filter system (Claret Medical Inc.) has two nitinol filters integrated in a steerable delivery system.17 The proximal filter is deployed in the brachiocephalic trunk and the distal one in the left common carotid artery, leaving the left vertebral artery unprotected. Three randomised trials evaluated subsequent generations of filterbased embolic protection. The Claret Embolic Protection and TAVI (CLEAN TAVI) study (NCT01833052) randomised 100 CoreValve TAVI patients to Sentinel embolic protection or no protection with brain MRI evaluation before TAVI and 2 days, 7 days and 30 days post TAVI.18 Patients with Sentinel protection had significantly fewer ischemic lesions and a smaller total lesion volume on days 2 and 7 post TAVI. The control arm also had more neurological changes, especially ataxia. The MRI Investigation in TAVI with Claret (MISTRAL-C) study (Nederlands Trial Register identifier NTR4236) enrolled 65 patients that were undergoing TAVI with either balloon-expandable or self-expanding transcatheter heart valves; patients had Sentinel protection or no protection.19 The trial suffered from a 40 % dropout rate for the follow up brain MRI study and ended up underpowered for its primary MRI endpoint. Still, patients with Sentinel protection had numerically fewer new lesions and a smaller total brain lesion volume. Effects were accentuated in the protected brain areas with up to half of the protected patients having no new lesions in these brain regions. Neurocognitive performance was also better preserved with Sentinel use. The SENTINEL Trial (NCT02214277) is the largest trial to date to address EPD with TAVI for general and filter-based protection, with the Sentinel in particular.20 The study enrolled 363 patients into a safety arm with Sentinel (n=123) and a 1:1 randomised comparison arm with (n=121) and without (n=119) Sentinel protection. Patients in the randomised arm had MRI scans at baseline and 2–7 days post-TAVI. Overall, use of Sentinel did not impact new lesion volume or neurocognitive performance. However, when adjusting for pre-existing lesion volume and transcatheter heart valve type in a post hoc analysis, Sentinel protection did significantly reduce new lesion volume. Moreover, there was a correlation between lesion volume and neurocognitive decline.
Histopathology
Filters deployed in the brain-supplying extracranial arteries can capture material travelling to the brain during TAVI and also offer the ability to be retrieved with the yield for histopathological analysis. Table 2 summarises published histopathology studies on this topic. The filters were safely used and retrieved in the majority of cases.17,19,21,23 Early use may have been hampered by a more challenging, less userfriendly first-generation filter design.
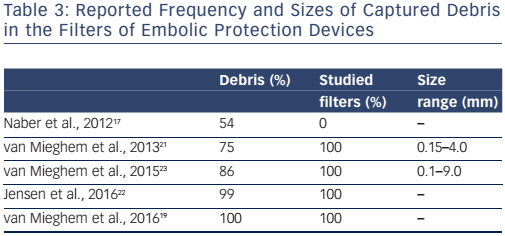
Across the studies, macroscopic debris was visible in 54–100 % of analysed filters and microscopic debris was present in more than threequarters of patients (see Table 3).17,19,21,23 Size of captured debris varied throughout the different studies, with the biggest piece being 9 mm.
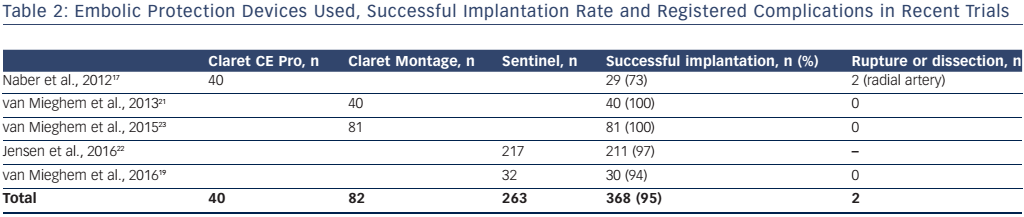
Fresh or organised thrombus was detected in >80 % of cases. Acute thrombus differs from organised thrombus and consists of platelets, fibrin, acute inflammatory cells, such as neutrophils, but no interspersed spindle-shaped cells.21 Acute thrombus is more frequent, with reported rates in TAVI patients as high as 86 % (see Figure 2).22
The frequency and origin of non-thrombotic tissue found in these studies was variable. Approximately one-third of patients had valve fragments in the filters, with sizes varying between 0.2 and 5.5 mm.19, 21–23 Degenerative aortic valve leaflets show distinctive features, including a central fibrous layer called lamina fibrosa, a proteoglycan-rich layer and surrounding fibroblasts (lamina spongiosa) and often contain amorphous calcified material at the base of the leaflets.23 Arterial wall fragments have be found in < 60 % of patients.22
Cardiomyocytes were identified in up to one-third of patients.19, 22, 23 Endothelial strands were responsible for the largest sized debris found in patients, with sizes varying from 0.2 to 9 mm in one-half to four-fifths of patients.19, 23 Foreign body material, derived from procedure-related fabric was found in 11–30 % of cases.19, 21–23 The Cerebral Protection in Transcatheter Aortic Valve Replacement (SENTINEL; NCT02214277) trial confirmed these findings with captured debris in 99 % of TAVI patients, most often ranging in size from 150 to 500 μm but occasionally greater than 1 mm.20
Discussion
The overall success and global adoption, particularly for lower risk patients, has meant TAVI-related procedural and long-term outcomes have been scrutinised. Increasing experience of practitioners and several astute device iterations has dramatically decreased the frequency of related paravalvular leakage, bleeding and vascular complications but not clinical neurological events.24 Most strokes occur within the first 48 hours after TAVI, but the stroke rate remains substantial throughout the first 2 months.7–9 History of cerebrovascular disease and procedural factors including valve embolisation or dislodgement and the need for post-dilatation are predictors for new neurological events after TAVI.7–9, 25 Approximately 50 % of strokes after TAVI are major strokes.7–9 Indeed, the randomised Placement of Aortic Transcatheter Valves II (PARTNER-II) intermediate risk trial reported a major stroke rate of 3.2 %, which is still comparable to the major stroke rates in the Placement of Aortic Transcatheter Valves I (PARTNER-I) and US CoreValve pivotal high-risk trials (3.8 % and 4.9 %, respectively).2, 3, 5 Major stroke is associated with 30-day (OR 7.43; 95 % CI [2.45–22.53]) and late (hazard ratio 1.75; 95 % CI [1.01–3.04]) mortality.7 A recent meta-analysis on the latest transcatheter heart valve designs still pointed towards a weighted 2.4 % stroke risk.26 Even more striking is the high incidence of brain injury after TAVI as demonstrated by DW-MRI.6 The majority of these brain lesions do not seem to cause immediate clinically apparent events, but the consequence of these subclinical brain lesions is unknown and there presence is, at least, suspicious. Indeed, silent infarcts have been linked to future clinical strokes, premature neurocognitive deterioration and dementia.10 Cerebral embolisation of thrombotic and tissue-derived material undisputedly occurs with nearly every TAVI procedure.19,20,21 Patients with advanced aortic valve disease have a higher risk for extensive and more complex atherosclerotic plaques in the aortic arch, which makes them more vulnerable to neurological events in general and after cardiac procedures in particular.27 CT analyses show larger atheroma volume to be a predictor for new cerebrovascular events, specifically when located in the ascending aorta and aortic arch.25 The combination of a diseased aortic wall together with a history of cerebrovascular disease (and thus greater atheromic burden) leaves TAVI patients more vulnerable to early and late strokes.25 Indeed, TAVI pertains wire and catheter navigation through diseased aorta’s, more or less difficult (retrograde) crossing of an aortic valve that is degenerated, thickened and contains calcifications, (acute or organised) thrombus, pannus and other friable tissue. The introduction, positioning and deployment of a transcatheter heart valve will displace the native diseased aortic valve, a process that may dislodge material. The seating of stiff wires in the left ventricle may scrape the myocardium. Repeated flushing and exchanging of catheters may also create gaseous and thrombotic embolisation.21 The mechanistic concept of embolic protection is intuitive and is supported by the recent literature. Debris is prevented from reaching the brain by deflection or filtering and may result in less and smaller brain injury with consequently preserved neurocognitive performance. Yet, the data to support EPD are still not compelling. So far EPD trials have been underpowered for their primary endpoints. Because the clinical neurological event rate after TAVI is sufficiently low and sample size in clinical trials with clinical endpoints would be unreasonably high, surrogate DW-MRI endpoints have been proposed. Yet assumptions of new lesion number and volume are challenging, with relatively limited benchmark data. MRI studies may also be aggravating to elderly TAVI patients, which is underscored by high dropout rates (≤40 %) in contemporary MRI follow-up studies.15,19 Patient and device selection may also affect the success of EPD. The DEFLECT-III and SENTINEL studies hinted towards more EPD benefit with self-expanding devices than with the balloon-expandable counterparts.15, 20 Conversely, in another study use of balloonexpandable (and not self-expanding) transcatheter heart valves was an independent predictor of tissue embolisation. A recent study-level meta-analysis of four randomised controlled EPD trials excluding SENTINEL, found significant reductions in total lesion volume and new ischemic lesions and more preserved neurocognitive performance in patients undergoing TAVI with EPD. 28 The high frequency of thrombotic material in the filters after TAVI may signal suboptimal per-procedural anticoagulation and may be addressed by improved anticoagulation regimens.21 Additional pooled-patient-level analyses of existing data and larger, adequately powered randomised trials should shed further light on whether TAVI in general would benefit from EPDs. In the meantime it is fair to claim that EPDs are safe and easy to use and will capture debris in nearly every TAVI procedure.