Kapil Lotun is Associate Professor of Medicine, Associate Director Cardiac Cath Lab, Director of TAVR Program, Director of Vascular Medicine, Director of Structural Heart Disease, Sarver Heart Center, Division of Cardiology, University of Arizona, USA.
The goals of therapy for cardiac arrest in the catheterisation laboratory are to maintain vital end organ perfusion and correct the precipitating cause of cardiac arrest, usually achieved by percutaneous coronary intervention (PCI). However, these goals often compete with each other. Manual chest compression is very challenging in the catheterisation laboratory, partly because of space limitations, and can result in the provider experiencing excessive radiation exposure. Mechanical cardiopulmonary resuscitation (CPR) provides unique advantages over manual chest compression for treating cardiac arrest in the cardiac catheterisation laboratory.1
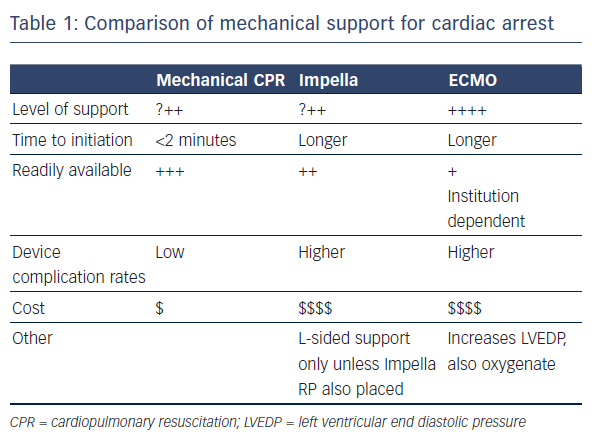
Mechanical circulatory support (MCS) has the potential to provide adequate end organ perfusion in this situation. It is readily available and can be initiated quickly. The available MCS devices have low complication rates and are inexpensive. Of the available devices, the TandemHeart is not practical to implant during cardiac arrest. Use of the Impella or extracorporeal membrane oxygenation (ECMO), however, hold more potential (see Table 1). A recent study found that the use of MCS during resuscitation of cardiac arrest in the catheterisation laboratory increases the rate of return of spontaneous circulation (ROSC).2 A case series (n=8) found that use of the Impella was feasible in selected patients with cardiac arrest and gave a 6-month survival rate of 50 %.3 The same survival rate was reported in a case series (n=14) that employed miniaturised ECMO systems.4
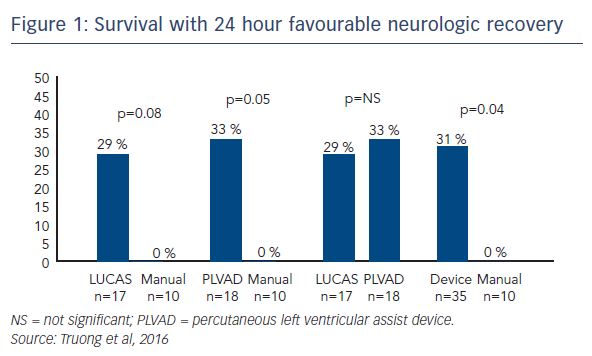
Recently, Dr Lotun’s team conducted a study in which 30 pigs were randomly assigned to interrupted manual chest compressions (n=10) versus either a piston chest compression device (LUCAS™; n=10) or a percutaneously inserted Impella device (n=10), supporting systemic haemodynamics and perfusion during two clinically relevant time periods of cardiac arrest associated with a left main/proximal left anterior descending (LAD) coronary occlusion in the cardiac catheterisation laboratory.5 The primary endpoint was favourable neurological function of survivors at 24 hours. Secondary endpoints included defibrillation success, ROSC and resuscitation-generated haemodynamics. The primary endpoint was achieved in 29 % of the LUCAS group and 33 % of the Impella group compared with none of the manual group.5 ROSC was achieved in 78 % of the Impella group, compared with 50 % and 59 % in the manual and LUCAS groups, respectively.
In conclusion, cardiac arrest in the catheterisation laboratory is a devastating event and will be more common with increasingly complex interventional procedures. The goal of circulatory support is to provide vital organ perfusion while the operator is correcting the underlying cause. Mechanical compression devices offer unique advantages over manual compression in this setting. The placement of percutaneous MCS devices can be considered but further studies are needed to define the optimal device and clinical outcomes.