The use of FFR guidance to identify and treat functionally significant coronary lesions has been shown to reduce the risk of MACE and save healthcare resources compared with angiographic guidance alone.4 Two types of FFR technologies are commercially available. Pressure wire technology involves a specially constructed 0.014" wire. Microcatheter technology employs a low-profile catheter with a pressure sensor incorporating fibre-optic technology into the distal end, giving a profile comparable to 0.022" diameter at the lesion site. The latter is more convenient and may overcome some of the limitations associated with conventional pressure wire systems, but the larger profile may influence coronary haemodynamics, an effect that might also depend on lesion and vessel characteristics. There is a need to investigate these unresolved issues in a large, multicentre, prospective study including a wide range of vessel and lesion types and utilising an independent core laboratory.
The ACIST-FFR prospective, open label study aimed to assess the differences between FFR measured by a microcatheter and commercially available 0.014" pressure wires in the setting of clinically relevant vessel diameters and lesion lengths.10,11 A Navvus microcatheter was used and compared to two different commercially available pressure wire systems: Abbott-St. Jude Medical and Philips-Volcano. The primary endpoint was the difference in agreement between microcatheter and pressure wire FFR as assessed by Bland-Altman analysis. The core laboratory (Cardiovascular Research Foundation, New York) assessed FFR and provided quantitative coronary angiography. Independent analyses were performed by Stanford University.
The inclusion criteria were patients with stable ischaemia; a vessel with a Thrombolysis in Myocardial Infarction flow of 3; a de novo lesion that a physician has considered to have a clinical indication for FFR measurement; and operator-assessed reference vessel diameter (RVD) of the target lesion ≥2.25 mm. Exclusion criteria were acute ST-elevation or non-ST-elevation MI, New York Heart Association Class 4 severe heart failure; a target vessel with an angiographically visible or suspected thrombus; a target lesion within a bypass graft; angiographic evidence of a dissection prior to initiation of pressure wire measurements and a target vessel containing excessive tortuosity or calcification.
The pressure wire was delivered, equalised and advanced across the lesion, a resting aortic pressure ratio (Pd/Pa) measured, then the Navvus microcatheter was advanced over the pressure wire, equalised, advanced past the lesion and Pd/Pa and FFR were measured simultaneously with both systems. The microcatheter drift was assessed; it was then withdrawn and FFR and drift were measured with the pressure wire alone.
The study enrolled 245 patients at 11 sites in the US. Of these, 13 subjects consented but FFR was not measured, FFR was measured but no drift assessed in nine and FFR was attempted but not measured in 13 because of failure to cross or device malfunction. The remaining 210 subjects underwent paired FFR assessment. Of these, 41 were excluded by the core laboratory either for ventricularisation, dampening of the proximal pressure tracings and drift from one or both of the systems without proper re-measurement. This left a primary cohort of 169 patients who met the core laboratory criteria.
The baseline patient characteristics were typical of PCI study populations: average age was 68 years and 37 % had a history of diabetes. Baseline angiographic characteristics were as follows: mean diameter stenosis was 47 %, i.e., angiographically indeterminate lesions. The average lesion length was 15.3 mm and the average RVD of the target lesion was 2.8 mm. Of the enrolled target lesions, 30 % were <2.5 mm in diameter. Therefore small vessels were well represented in this study – prior microcatheter FFR clinical studies had only included vessels with RVD ≥2.5 mm. The mean pressure wire FFR was 0.83, again representative of a patient population with a clinical indication for FFR.
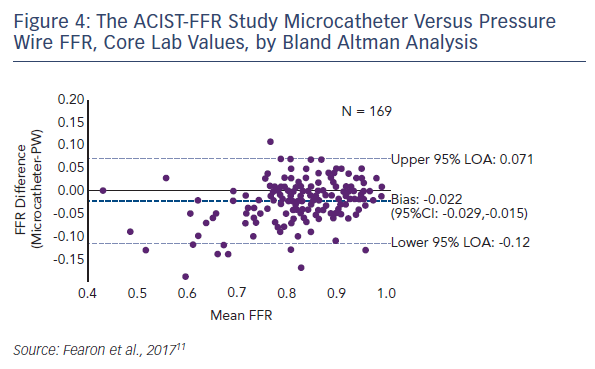
Compared with the pressure wire, the microcatheter gave consistent and modestly lower FFR measurements with an average bias of −0.022 (see Figure 4). In terms of the secondary endpoint, the difference in pressure wire FFR with and without the microcatheter on the pressure wire, the average bias was −0.03. A sensitivity analysis of all site-reported data (i.e., all patients with paired FFRs) showed that the bias was similar to that of the primary analysis cohort (−0.025). A strong correlation was seen between the microcatheter and the pressure wire FFR (Pearson coefficient 0.901, p<0.001). Since the bias is less at FFR values within the grey zone and higher, the clinical impact of the difference may be minimal. For a threshold of 0.80 or less, the diagnostic agreement was 81 %. There were only five cases (2.9 %) where the pressure wire FFR exceeded 0.80 but the microcatheter FFR was <0.75.
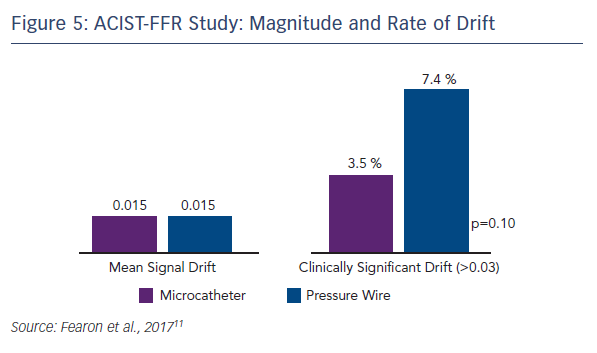
Because of the large study cohort, it was possible to identify predictors of bias. On univariate analysis, smaller RVD tended to predict greater bias and longer lesion length was a significant predictor of greater bias. However, using multivariate analysis incorporating microcatheter FFR, only the FFR measured by the microcatheter was found as the independent predictor of bias. Mean signal drift was similar between the two technologies, but clinically significant drift (defined as >0.03) was numerically higher with the pressure wire (7.4 % vs. 3.5 %, p=0.10; see Figure 5).
In summary:
- A pressure-monitoring microcatheter provides a modestly lower FFR value compared with a traditional pressure wire, with an average bias of −0.02.
- Using univariate analysis, smaller RVD and greater lesion length tend to predict a lower FFR measured by the microcatheter.
- Using multivariate analysis, lower FFR as measured by the microcatheter is the only predictor of a greater difference between the two measurements.
- Since the bias is less at higher FFR values (e.g., the grey zone and above), the clinical impact of this difference for those scenarios is likely minimal.
This study confirms and expands previous findings and provides robust evidence for the diagnostic capabilities of microcatheter FFR.