Transcatheter aortic valve implantation (TAVI) is included in guidelines for the treatment of severe symptomatic aortic stenosis (AS) in patients with intermediate to high risk for standard surgical therapy.1 The gender-related difference in the pathophysiology of heart disease (and consequently in its clinical course and treatment) has already been identified as an issue in interventional cardiology.2 Women are commonly underrepresented in coronary trials, but they constitute nearly half of the population assessed in clinical trials evaluating TAVI.3–5 This raises the need to study the impact of gender, because it is still debated whether sex-specific factors influence and modify the clinical course of AS over time and whether hormonal changes, including a history of pregnancy and age of menopause, can impact TAVI outcomes after implantation.
Female sex is an independent risk factor for mortality after surgical aortic valve replacement and hence is included in the Society of Thoracic Surgeons (STS) risk score model.6 Insights from the Placement of AoRtic TraNscatheTer Valve (PARTNER) trial showed that TAVI may be preferred over surgery for female patients due to a lower rate of late mortality in the TAVI arm.7 Subsequent observational studies and meta-analyses have compared outcomes after TAVI but have found conflicting results in terms of 30-day mortality: some studies did not find any significant difference between the sexes, while others suggested a higher mortality among males.8–11
The aim of this article is to review the literature on this topic, to provide a complete analysis of what is known about female patients undergoing TAVI and to clarify the role of potential sex-specific predictors of the main endpoints after TAVI, as defined by the Valve Academic Research Consortium (VARC)-2 criteria.12
Method
We searched on PubMed for publications that included the following words in the title: ‘transcatheter aortic valve implantation/replacement (TAVI/TAVR)’, ‘sex/gender’, ‘women’ and ‘outcomes’. References from reviews, registries and meta-analyses were also examined for potentially relevant citations. Articles written in languages other than English, case reports and abstracts were excluded from the analysis.
We looked for gender differences in baseline clinical characteristics, imaging findings and in procedural aspects. We then analysed both short- and long-term outcomes. Reported rates of procedural success and main complications, including mortality, were recorded. We also searched for probable sex-specific predictors of outcomes described in the literature.
Baseline Characteristics
Clinical Characteristics
Women with severe AS are older than men at presentation. In observational studies performed in the surgical era, this finding was ascribed to a lower awareness of heart disease among women. It was considered that this lack of awareness led women to underestimate their condition and hence seek medical attention later, resulting in a possibly negative impact on outcomes.13 More recent meta-analyses of TAVI studies have confirmed that women are still older than men at presentation, not only for cultural reasons but also because they become symptomatic later.14 Indeed, a different left ventricular response to pressure overload may be involved, as discussed later.
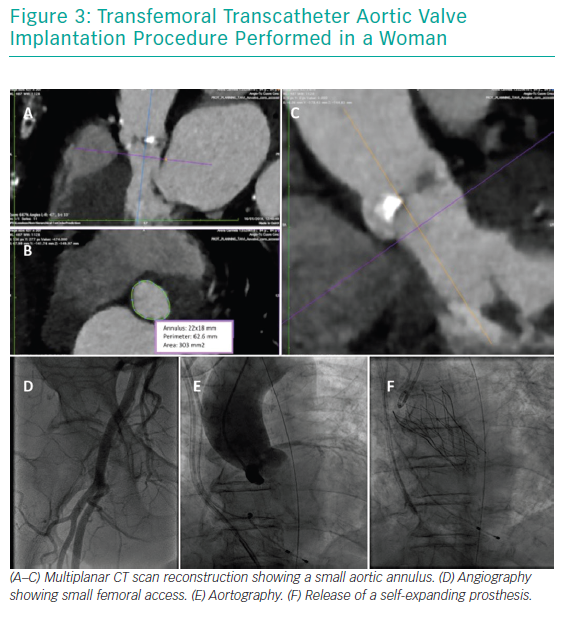
Women with severe AS are generally ‘healthier’ than men in terms of baseline comorbidities (Table 1). They have a lower prevalence of risk factors, particularly diabetes, as well as concomitant coronary artery disease, history of previous MI and peripheral vascular disease.15 This translates into lower preoperative risk scores for women presenting for TAVI in comparison to men.14 However, even if women are usually healthier than men on presentation, the presence of particular comorbidities can worsen their outcomes after TAVI. For example, a positive history of coronary artery disease at baseline, with or without recent coronary intervention, is associated with adverse 1-year outcomes.16 Similarly, baseline AF is a predictor of increased all-cause and cardiovascular mortality at 12 months (adjusted HR 1.67 and 1.85, respectively) in multivariate analysis.17
Echocardiographic Findings
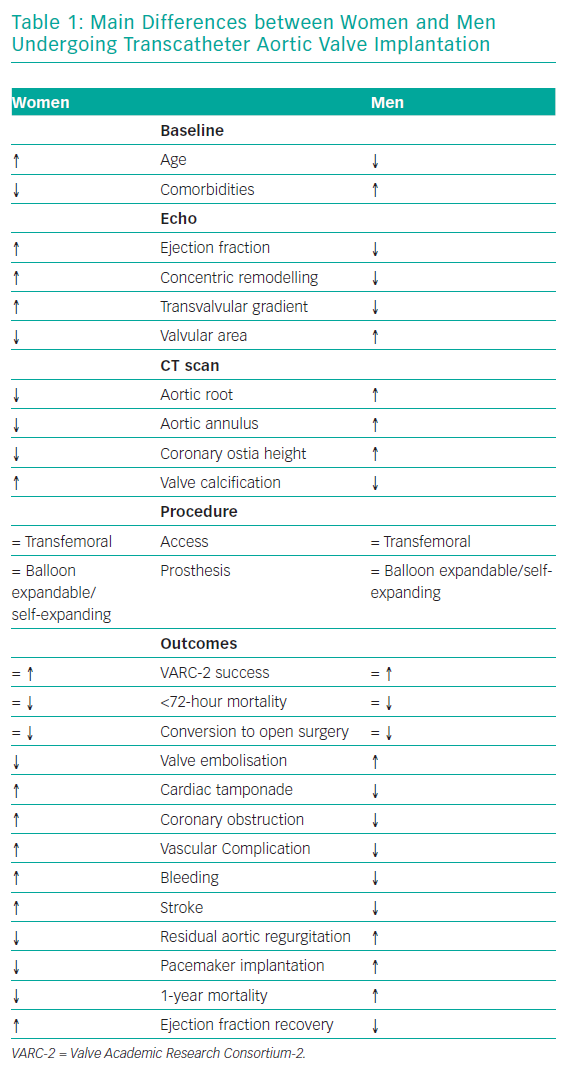
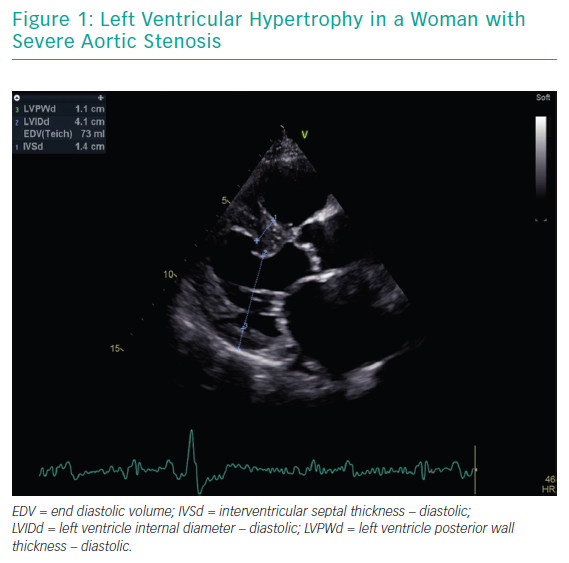
The most striking sex-related difference in echocardiographic findings before TAVI is that women usually have a higher left ventricular ejection fraction than men.18,19 Better left ventricular ejection fraction is mainly associated with a lower extent of myocardial fibrosis, potentially indicating a lower burden of irreversible myocardial damage.20 Myocardial response to pressure overload is different in women and men. Female patients have a higher prevalence of concentric remodelling (Figure 1) while male patients show a higher rate of maladaptive remodelling that eventually leads to left ventricle dilation (Figure 2). For this reason, women more frequently present with paradoxical low-flow low-gradient severe AS, while low-flow low-gradient severe AS is more common in men.21 Although both groups have poor outcomes in comparison to patients with normal-flow high-gradient severe AS, low-flow low-gradient severe AS confers the worst prognosis, partially explaining the post-TAVI survival benefit seen in women. Another difference between men and women is that the latter more commonly have worse AS with higher transaortic gradients (mainly related to their better preserved ejection fraction), smaller aortic valve areas and higher pulmonary pressures.19
CT Scan Findings
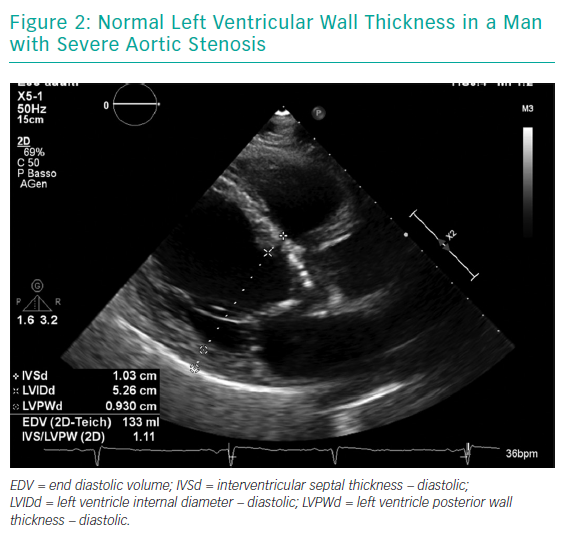
Gender differences are also present on CT scans (Figure 3). Women have smaller aortic root dimensions (even after indexing for their smaller body surface area) and their left and right coronary artery ostia are positioned lower than in men. These features might increase the risk of coronary obstruction after TAVI. Conversely, absolute smaller sizes of aorta, subclavian and iliofemoral arteries are not confirmed after indexing for body size.22
Another distinctive feature highlighted on CT scans is that women have smaller aortic annuli.23 This has been related to a higher rate of patient–prosthesis mismatch in women undergoing surgery, but is significantly lower in female patients undergoing TAVI.24,25 This is mainly due to differences in TAVI valve design, such as the supra-annular location of some valve prostheses and the absence of a sewing ring. The lower incidence of patient–prosthesis mismatch in TAVI patients might make this intervention preferable to surgery in female patients with small aortic annuli.
Finally, it has been demonstrated that the total burden of aortic valve calcification for any given level of AS severity is significantly lower in women than in men; thus, the Agatston coronary artery calcium score cut-off points are 2,065 in men and 1,275 in women.26 These scores are usually used to aid diagnosis in patients with discordant aortic valve area and mean transaortic gradient values. Furthermore, the use of ‘calcium density’ has been shown to be predictive of survival in AS patients independently of clinical and echocardiographic factors.27 The minority of women who show moderate to severe calcifications of the left ventricle outflow tract have an almost two-fold increased risk of mortality or stroke at 1 year.28 In addition, calcium volume in the right coronary cusp is an independent predictor of the need for pacemaker implantation post TAVI.28
Procedural Aspects
Access
Transfemoral approach is the preferred route of access in the general population undergoing TAVI, particularly in women. However, women have a higher rate of vascular complications following TAVI. This is usually due to an unfavourable sheath-to-artery ratio in women, as the common femoral arteries have a smaller mean diameter and there is increased tortuosity in comparison to men.14
Prosthesis
There are conflicting data about the types of valve implanted in female patients. The meta-analysis by O’Connor et al. including data from five studies and 11,310 patients showed that balloon-expandable (BE) devices were used more frequently and that a higher proportion of women than men received a BE prosthesis.14 In this series only Sapien® and Sapien XT® (Edwards Lifesciences), CoreValve™ (Medtronic) and Portico™ (St. Jude Medical) valves were used.
An even larger meta-analysis by Saad et al. including 47,188 patients confirmed that women were more likely to receive a BE valve compared with men (76.2±38.7% versus 72.7±38.7%; p=0.0001).29 The more frequent use of BE prostheses in women might partially explain the higher incidence of annular rupture, with further contributing factors being smaller annular and aortic root dimensions in females in comparison to males. It might also explain the lower incidence of atrioventricular block requiring permanent pacemaker implantation as well as paravalvular aortic regurgitation (as shown in the next section).
However, a possible confounder is these meta-analyses included many studies referring to initial TAVI experiences, when a reduced armamentarium of devices was available. Consequently, the more common use of BE devices in women at that time can be interpreted as being proportionate to their less frequent employment in men, in whom only self-expanding valves could fit the larger annular sizes.
A more recent sex-specific registry by Chieffo et al., which enrolled 1,000 women from 19 countries across Europe and North America, found a wider range of devices have been used since the introduction of new-generation prostheses. In this changed scenario, self-expanding (SE) CoreValve prosthesis are now the most commonly used devices (47.2%) while transfemoral is still the preferred route of access.30 The preference for using SE devices in women and patients with smaller annuli and calcified iliofemoral vessels was confirmed by a recent single-centre US study of third-generation devices, reflecting real-world practice.31 The supra-annular design of some of these prostheses may have contributed to this changing scenario as it favours lower post-procedural gradients, thus reducing the incidence of patient–prosthesis mismatch.
Outcomes
Procedural Outcomes
O’Connor et al. reported a high device success rate (97%) in the whole TAVI population without any gender-related difference (p=0.22), together with a comparable incidence of immediate (<72-hour) peri-procedural mortality (2.2% versus 2.6%, p=0.24) and need for conversion to open surgery (0.9% versus 1.0%, p=0.57). Conversely, valve embolisation is slightly more common in men (1.5% versus 1.0%, p=0.018) while cardiac tamponade has a significantly higher incidence among women (0.7% versus 1.3%, p=0.002).14
Coronary obstruction is a rare but life-threatening complication after TAVI, more often involving the left coronary system. It has been shown to be more frequent in elderly patients, in patients receiving a BE valve, in those with a previous surgical bioprosthesis and, most of all, in women (p<0.001).32 One possible explanation for this finding is the lower coronary artery height observed in women. Other peri-procedural endpoint results seem to be consistent, even if there is some heterogeneity in definitions (VARC criteria were applied in most but not all studies).
Despite lower rates of baseline peripheral vascular disease, women are more prone to vascular complications and subsequent bleeding.8,9,33 Female sex is associated with a 1.72-fold increase in major vascular complications, as defined by Stangl et al. in their meta-analysis of 14 studies and 7,973 patients.33 Both Zhao et al. and O’Connor et al. confirm this result and show that women are at a higher risk of major/life-threatening bleeding (15.0% versus 12.7% and 10.5% versus 8.5%, respectively).11,14 This finding has historically been ascribed to the smaller absolute dimensions of the iliofemoral arteries in women, implicating an unfavourable introducer sheath-to-femoral artery ratio.34,35 Technical improvements in TAVI procedure have aimed to overcome this problem.
Gender difference in the prevalence of peri-procedural stroke is controversial: some studies consider the incidence to be equally low in both sexes but several studies report a higher stroke rate in women.14,36 An analysis of data from the STS/American College of Cardiology Transcatheter Valve Therapies Registry showed that, compared to men, women have a higher risk of stroke (HR 1.40).37 The finding of a higher incidence of stroke in women in some studies is surprising, since women have fewer comorbidities at baseline. The use of a large sheath or the distribution of ascending aortic atheroma might explain these unexpected findings.37
Other important determinants of outcomes after TAVI are the presence of residual aortic regurgitation (AR) and the need for permanent pacemaker implantation. Both are less common in women than in men. The incidence of post-procedural AR grade ≥2 (where grade 2 is mild) is 20.9% in women and 29.6% in men (p=0.01). This is due to women having a smaller annulus and higher cover index, which is calculated as 100 × ([prosthesis diameter − trans-thoracic echocardiography annulus diameter]/prosthesis diameter).38
Permanent atrioventricular block is similarly less common in female than male TAVI patients (9.6% versus 17.2%). The Italian OBSERVANT Registry reported a trend towards significance for female sex as a protective factor (p=0.05; OR 0.61).39 Results are even stronger if focusing on studies in which CoreValve use is dominant, such as the meta-analysis by Zhao et al.11 One suggested explanation for the higher incidence of permanent pacemaker implantation in men as compared to women is the use of larger prostheses.
Long-term Outcomes
Although some small studies have shown similar survival between the sexes,9,40,41 large meta-analyses have consistently demonstrated a survival benefit for women at mid-term follow-up after TAVI.17
For example, Stangl et al. and Conrotto et al. assessed mortality and showed that it was higher in men than women at 3 months (25.9% versus 19.7%) and 1 year (34% versus 24%).33,36 The STS/American College of Cardiology Transcatheter Valve Therapies Registry also demonstrated that male sex was significantly associated with 1-year mortality (HR 1.21), together with other risk factors such as advanced age, end-stage renal disease, severe chronic obstructive pulmonary disease, non-transfemoral access, STS score >15% versus <8% and preoperative AF/flutter.37
Similarly, the meta-analysis by O’Connor et al. confirmed a higher mortality for men in comparison to women at a median follow-up of 387 days (21.8% versus 17.3%, respectively). Furthermore, it showed that female patients have a better prognosis on longer-term follow-up (up to 5 years, when the percentage survival is 43.6% in women versus 38.9% in men). Multivariate analysis also found that female sex is a predictor of better outcome, irrespective of the valve type and the route of access.14
Different outcomes between women and men undergoing TAVI can be explained by a complex interaction between baseline characteristics and procedural aspects. The healthier state of female patients at baseline, including better ventricular function, seems to be of primary importance. In men, a higher incidence of coronary artery disease and the consequently wider extent of myocardial fibrosis partially explain this finding.42 Moreover, a lack of oestrogen and high levels of circulating androgens in males contribute to the increase in interstitial fibrosis through up-regulation of the extracellular matrix.43 As a consequence, when exposed to pressure overload due to AS, male hearts develop systolic dysfunction and heart failure earlier than female hearts, which exhibit a more favourable geometry for the preservation of systolic pump performance.43–45
The close involvement of sex hormones in cardiac function has been demonstrated in an animal model. Gonadectomy in rats significantly reduces cardiac function, inducing a shift in myosin heavy-chain content to the slower isoform; a pathological effect that can be reversed by hormonal supplementation.46
The combination of all these factors can also directly influence response in terms of recovery of ejection fraction after aortic valve replacement. Morris et al. showed that women have an earlier improvement in ventricular function than men.47
Gender-specific analysis
The Women’s International Transcatheter Aortic Valve Implantation (WIN-TAVI) Real-World Registry found that increasing age, history of prior stroke, left ventricular ejection fraction <30% and the generation of TAVI device deployed were all independent predictors of 30-day VARC-2 primary safety endpoint (a composite of all-cause mortality, stroke, major vascular complication, life-threatening bleeding, acute kidney injury, coronary artery obstruction and repeat procedure for valve-related dysfunction) in women.48 In the effort to identify sex-specific factors that may influence outcomes, a history of pregnancy was found to be protective (OR 0.63). Age of menopause, history of osteoporosis and gynaecological or breast cancers seemed to have no influence on the primary safety endpoint.29 The updated 1-year follow-up demonstrated a univariate but not multivariate protective association of previous history of pregnancy with 1-year incidence of death or stroke; also it failed to demonstrate any association with the VARC-2 composite efficacy endpoint beyond 30 days (a composite of all-cause mortality, all stroke, MI, hospitalisations for valve-related symptoms or worsening congestive heart failure or valve-related dysfunction).48
Conclusion
Percutaneous rather than surgical aortic valve replacement is better for women with AS. The potentially negative impact of some procedural complications in women is counterbalanced by the healthier state of the female population at baseline and the lower incidence of post-procedural AR and permanent pacemaker implantation. Together these findings lead to the survival benefit observed in women. A history of pregnancy has a protective role against adverse outcomes occurring within 30 days of procedure but has no influence on outcomes in the longer term.