According to the International Diabetes Federation, 382 million people had diabetes in 2013, with diabetes mellitus (DM) accounting for around 90 % of all diabetes cases. By 2025 the number will rise to 592 million.1 The association between DM and cardiovascular disease is well established2 and percutaneous coronary intervention (PCI) is associated with worse clinical and angiographic outcomes among the diabetic patient population than among non-diabetics.3 Despite improvements in interventional techniques and advances in stent technology, this difference in outcomes persists. There is a greater likelihood of restenosis after PCI with drug eluting stent (DES) implantation in diabetic patients, particularly occlusive restenosis.4,5 There is also a higher risk of adverse cardiac events in diabetic patients who undergo DES implantation compared with those who undergo coronary artery bypass grafting (CABG).6–8 Second-generation DES provide superior safety and efficacy results compared with first generation DES and have been successfully used in diabetic patients,9–12 but unmet needs remain in coronary interventions in this growing patient population, leading to the hypothesis that patients with DM need a specialised DES. This article aims to review the clinical evidence in support of the Cre8™ DES in patients with DM.
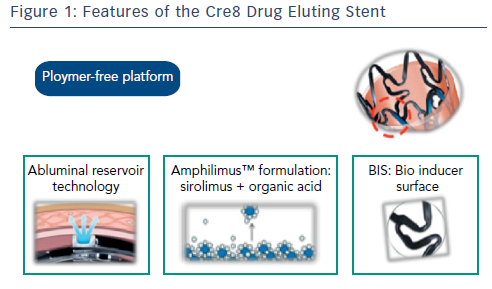
The Cre8 Drug Eluting Stent
The Cre8 DES is polymer-free, eliminating the disadvantages associated with durable and absorbable polymers or their breakdown products. Its drug release system (Abluminal Reservoir Technology) features reservoirs on the outer surface of the stent. These enable controlled drug elution that is directed towards the vessel wall (see Figure 1). The Amphilimus™ carrier, comprising sirolimus and an organic acid, enhances drug bioavailability.13 Its optimised permeability allows a homogeneous distribution inside the vessel wall and a uniform action on the whole tissue, enabling an optimal balance between safety and efficacy. The effects of sirolimus are dose dependent; in diabetic patients, more sirolimus is needed to achieve the equivalent anti-proliferative action in healthy cells.14 The organic acid of the Amphilimus formulation increases the permeation of the sirolimus into the cells of diabetic patients. After drug release, a bare metal stent (BMS) remains. The Cre8 DES is covered with a bio inducer surface made of pure carbon, which is biocompatible and does not produce any late inflammatory stimuli inside the treated segment, reducing the inflammatory response and lowering the risk of stent thrombosis.
Clinical Studies Evaluating the Efficacy and Safety of the Cre8 Stent
Initial clinical data in support of the efficacy of the Cre8 DES came from the International randomised comparison between DES Limus Carbostent and Taxus drug-eluting stents in the treatment of de novo coronary lesions (NEXT) clinical study, which demonstrated the noninferiority of the Cre8 DES versus a permanent-polymer paclitaxeleluting stent. The primary endpoint was 6-month angiographic in-stent late lumen loss (LLL). The most striking finding of this study was the finding that the LLL in the diabetic subgroup was comparable to that in the general study population, a finding that had not been seen with other DES.15 Following the demonstration of efficacy and safety in the NEXT clinical trial, the next steps in the Cre8 clinical development program included two real-world studies. The first was the MultIceNtric and RetrospectiVe REgiStry in ‘real world’ paTients with polymer-free drug elutInG stent CRE8 (INVESTIG8), which features diabetic and non-diabetic participants. The second is an ongoing realworld study in the diabetic population and Prove Abluminal Reservoir Technology Clinical benefit in all comers patients (pARTicip8).
The INVESTIG8 study had a primary endpoint of the incidence of a clinical composite endpoint (cardiac death/target vessel myocardial infarction [MI] target lesion revascularisation [TLR]) from the index procedure to 12 months. Secondary endpoints included the incidence of a clinical composite endpoint (all deaths/all MI/ any revascularisation) from the index procedure to 12 months; and the incidence of stent thrombosis from baseline procedure to 12 months, classified according to the Academic Research Consortium definition. A data sub-analysis presented at EuroPCR 2015 and not yet published indicates that at 1 year the composite endpoint was observed in 3.5 % of the non-diabetic population and 5 % of those with diabetes. Freedom from events was reported in 97.8 % of the non-diabetic study population and 95.6 % of those with diabetes. Definite and probable stent thrombosis occurred in only 0.6 % of the non-diabetic and 1.4 % of the diabetic population.16
The RESERVOIR Clinical Study
Following the promising findings of the NEXT and INVESTIG8 studies, a clinical trial, the Randomised Comparison of Reservoir-based Polymer-free Amphilimus-eluting Stents (AES) versus Everolimus- Eluting Stents (EES) in Patients with Diabetes Mellitus (RESERVOIR) focused on patients with DM.17 A meta-analysis of 42 trials comparing sirolimus, paclitaxel, everolimus and zotarolimus-eluting DES in diabetic patients, with 22,844 patient years of follow-up, concluded that there was no increased risk of any safety outcome (including very late stent thrombosis) with any DES compared with BMS, and that the EES was the most efficacious and safe of available DES.18 The study concluded that there was an 87 % probability that EES are the most efficacious DES compared with the others and a 62 % probability that they were the least likely to develop stent thrombosis. Therefore the EES was chosen as the control treatment in the RESERVOIR study.
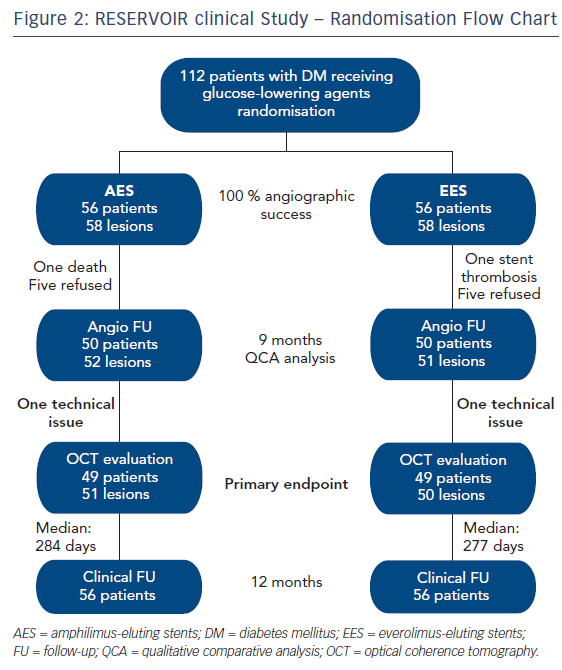
The study population comprised patients with DM treated with glucose-lowering agents requiring PCI of a native coronary artery. Exclusion criteria included glycaemic control by lifestyle changes only, prior ST-elevation myocardial infarction (STEMI), presence of a bifurcation >2.5 mm, target vessel left main/ostial left anterior descending (LAD) artery and/or ostial left circumflex artery with indication for CABG, glomerular filtration rate <30 ml/min/1.73 m2, left ventricular ejection fraction <30 % and contraindications to dual antiplatelet therapy (DAPT) for 12 months. Patients (n=112) were randomised either to an AES (Cre8) or an EES (Xience). Angiographic follow up was performed at 9 months with qualitative comparative analysis (QCA). The primary endpoint was neointimal hyperplasia volume obstruction, as assessed by optical coherence tomography at 9 months (see Figure 2).17
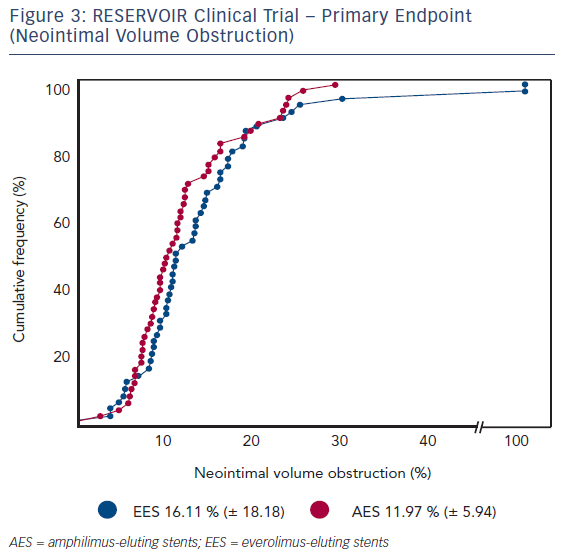
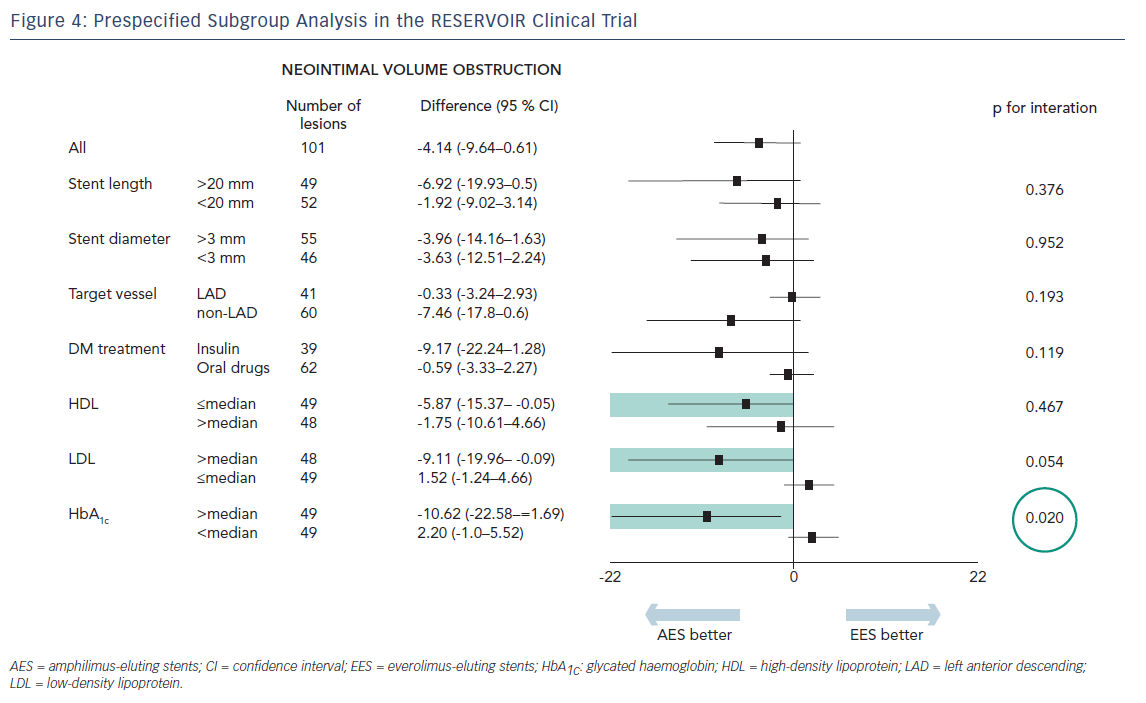
Mean glycated haemoglobin (HbA1c) at baseline was 7.5 % ± 1.2 % across both treatment groups. Insulin was used for diabetes control in 21 (37.5 %) and 24 (42.9 %) patients in the Cre8 and Xience treatment groups, respectively. Before the index procedure, reference vessel diameter (RVD) was 2.69 ± 0.54 mm and 2.55 ± 0.49 mm for the Cre8 and Xience groups, respectively. The acute gain in-stent was 1.59 ± 0.40 mm (Cre8) and 1.54 ± 0.43 mm (Xience); in-segment was 1.22 ± 0.46 mm and 1.17 ± 0.60 mm for Cre8 and Xience groups, respectively.The primary endpoint, neointimal volume obstruction was seen in 11.97 ± 5.94 % of patients in the Cre8 group and 16.11 ± 18.18 % of patients in the Xience group (p for noninferiority = 0.0003; p for superiority = 0.22; see Figure 3). Prespecified subgroup analysis showed that the superior performance of the Cre8 was demonstrated across all subgroups. In addition, the Cre8 was statistically superior in the subgroup in which HbA1c levels were above median – see Figure 4.
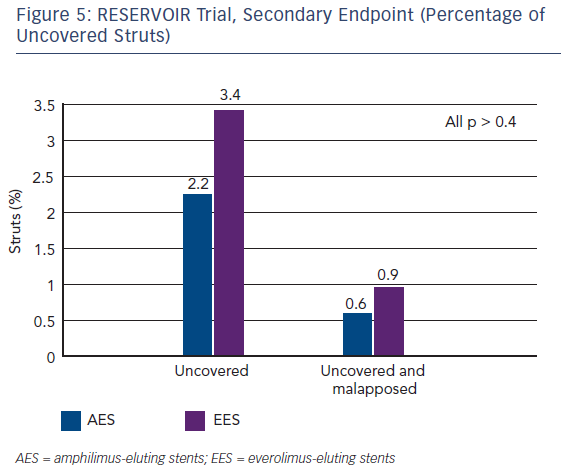
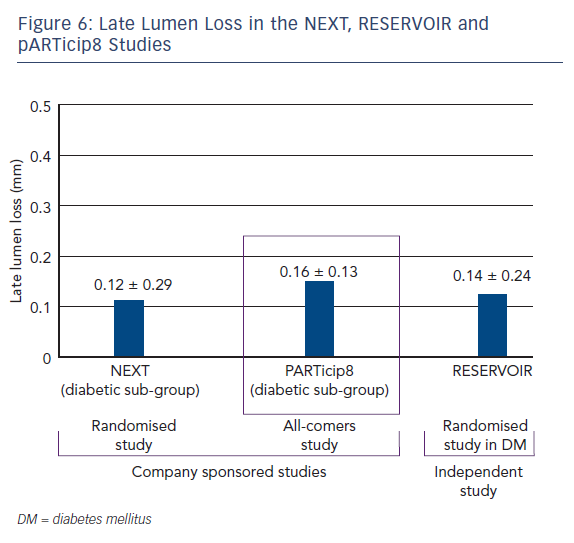
In terms of secondary endpoints, the percentage of uncovered struts was similar in both patient groups (see Figure 5). The Cre8 also showed noninferiority in minimal lumen diameter and diameter stenosis. The two groups also showed similar findings in terms of clinical events at 12 months. In addition, the LLL in the Cre8 was 0.14 ± 0.24 mm compared with 0.24 ± 0.57 mm in the Xience. The Cre8 LLL was also evaluated in the NEXT clinical study.15 Also noteworthy is the low standard deviation of the Cre8 compared with the Xience, suggesting that data from the Cre8 are highly consistent. This can be explained by the amphilimus formulation, which increases the sirolimus permeation into the cells.
The PARTicip8 Study
The pARTicip8 clinical observational prospective study recruited 1,186 ‘real world’ patients with ischaemic myocardial symptoms related to de novo lesions in native coronary arteries, in 30 European sites.19 One hundred patients from a pre-specified diabetic subgroup were submitted to angiographic follow-up. The primary endpoint was a composite of cardiac death, target vessel MI and CI TLR at 6 months from the index procedure. Secondary endpoints included incidence of the endpoint at 30 days, one year and yearly up to 5 years, stent thrombosis and angiographic measurements in-stent and in-segment. PARTicip8 data presented at the Transcatheter Cardiovascular Therapeutics annual meeting in 2015 show that the primary endpoint, device oriented major adverse cardiac event (MACE) was observed in 2.0 % (95 % confidence interval [CI] [1.26 – 2.95 %]) in the overall population and 3.4 % (95 % CI [1.63 – 6.13 %]) in the diabetic population.19 Of note was the CI TLR at 6 months: 0.5 % in the overall population and 0.78 % in the diabetic subgroup. The LLL in the diabetic subgroup was 0.16 ± 0.13 mm, a similar value to those obtained in the NEXT and RESERVOIR studies (see Figure 6). At 1 year the device oriented MACE was reported in 2.8 % of the overall population and 4.7 % of the diabetic subpopulation, the CI TLR was 1 % in the overall population and 1.4 % in the diabetic subgroup. These findings add to the growing body of clinical data in support of the Cre8 DES and demonstrate efficacy and safety in real world use.
Conclusion
Patients with DM undergoing coronary interventions remain at high risk for stent restenosis and adverse cardiovascular events despite the availability of second generation DES. Clinical trials and real-world data show that the formulation of the antiproliferative drug with an amphiphilic carrier in the Cre8 stent could result in improved efficacy in those challenging patients. The Cre8 DES has demonstrated remarkable consistency in terms of LLL across three clinical studies. The RESERVOIR clinical trial is the first study to show statistical superiority of Cre8 in diabetics patients with HbA1c >7.3 %. These data and the findings from other clinical and real-world studies, suggest a high efficacy of the Cre8 in patients with DM. A larger clinical trial is warranted to confirm these important findings.