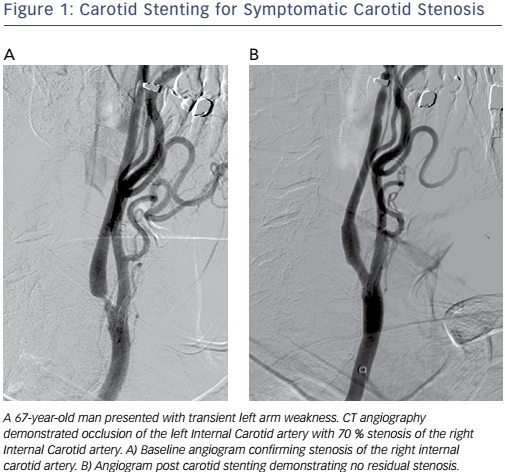
Catheter-based vascular interventions continue to evolve as new devices continue to expand the capabilities of interventionalists and improve patient safety. The importance of athero-embolisation during vascular intervention has long been recognised.1 Embolic protection devices (EPDs) were developed to help prevent embolisation during endovascular procedures. The risk of distal embolisation is considered significant in the carotid arteries (see Figure 1), saphenous vein grafts and thrombotic lesions affecting patients with acute coronary syndromes.2 While EPDs have been designed and clinically tested for these procedures, their use during procedures in the other vascular territories has been questioned because of the increased cost, potential risk of complications and perceived lack of significance of distal embolisation in these vascular beds.3
Embolic Protection Devices
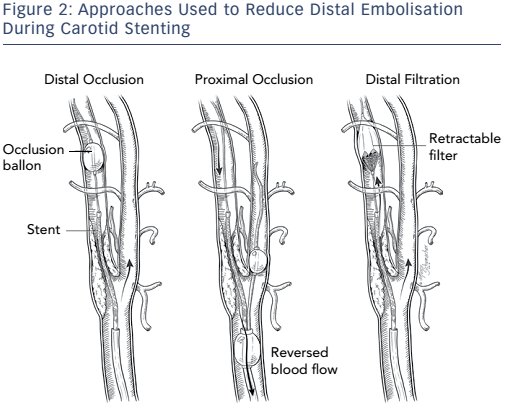
Different approaches have been used in attempts to reduce distal embolisation during vascular interventions (see Figure 2). Current technologies for embolic protection employ three basic strategies:
- temporary flow arrest using an occlusion balloon distal to the lesion being treated;
- placement of a temporary filter distal to the lesion being treated while maintaining antegrade flow; and
- proximal occlusion of the vessel, with and without flow reversal. Each technique has its advantages and disadvantages.
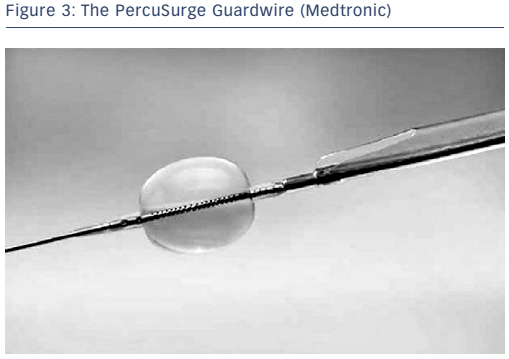
The concept of flow arrest for distal protection during carotid artery stenting was first described by Theron and colleagues.4 Distal occlusion temporarily arrests antegrade blood flow while the intervention is performed. The PercuSurge Guardwire® (Medtronic; see Figure 3) is a commercially available device with a balloon mounted on a wire that is advanced distal to the lesion and inflated. An aspiration catheter is placed over the wire after the intervention and any debris aspirated. The advantage to this strategy is that debris trapped in the stagnant column of blood can be aspirated before the occlusion balloon is deflated. Known complications include vasospasm and vessel dissection. Disadvantages of distal protection with occlusion balloons include: ischaemia within the territory of the occluded artery, the complete occlusion of flow making it difficult to see the lesion, the possible formation of thrombus distal to the balloon due to the low flow state, and the potential for allowing embolic debris along the edge of the balloon to go downstream once the balloon is deflated or vessel expansion occurs due to dilation from angioplasty or stenting. In the cerebrovascular system, approximately 5 % of patients do not tolerate balloon occlusion.5
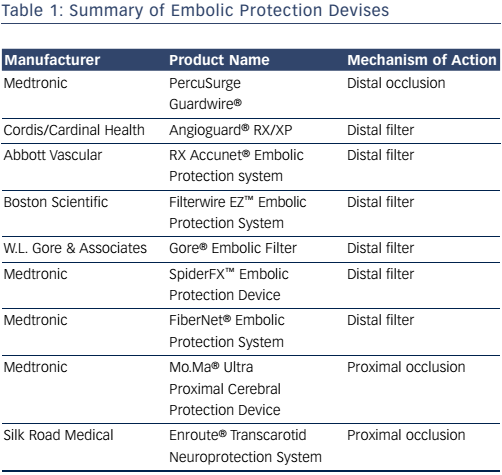
Devices that maintain antegrade flow consist of a filter basket attached to a guidewire that captures embolic debris while maintaining antegrade flow – a filterwire. There are many filter-based EPDs currently available (see Table 1). These devices consist of a membrane with 80–130 um pores. These devices do not offer the same protection as an occlusion balloon, as smaller particles can still pass through the filter. The filter is attached to 0.014” guidewire, which is used as the guidewire during the procedure. The filter is constrained in a catheter and advanced and opened distal to the lesion. After the procedure, a retrieval catheter is advanced over the wire closing the filter and trapping the debris. The SpiderFX™ (Medtronic; see Figure 4) is unique in that it is loaded into a delivery catheter that is advanced distal to the stenosis over a second 0.014” guidewire of the operators choosing, with subsequent deployment of the filterwire. The Abbott Vascular Emboshield® NAV6 filter is not fixed to the wire and allows for limited wire movement independent of the filter. Filter devices have a larger crossing profile than the PercuSurge Guardwire. Disadvantages include the passage of small particles and complications related to advancement, deployment and recovery of the filters.
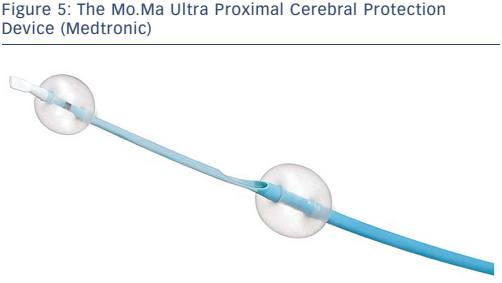
Proximal occlusion devices work by stopping or reversing the antegrade flow of blood. These devices have the advantage of protecting the distal circulation prior to crossing the lesion; the distal protection devices must be advanced across the lesion prior to deployment, thus risking dislodgment of debris prior to deployment of the EPD. Devices designed specifically for the cerebrovascular circulation consist of a balloon guiding catheter for occlusion of the proximal common carotid artery (CCA). A second balloon catheter is incorporated into the guiding catheter to occlude the external carotid artery (ECA). The ECA balloon is inflated first to prevent retrograde flow in the ECA from providing collateral flow and allowing persistent antegrade flow in the internal carotid artery (ICA) after the CCA balloon is inflated. The Mo.Ma® Ultra Proximal Cerebral Protection Device (Medtronic; see Figure 5) is the only currently available device in the US designed for percutaneous use. Debris can be aspirated from the guiding catheter in the CCA during the intervention. The theoretical advantage of proximal protection is the ability to arrest or reverse flow prior to crossing a carotid stenosis. This should provide a maximal means of protection. Unfortunately, these devices are large, requiring a 9-french (Fr) sheath. As such, they can be difficult to navigate around a difficult arch. As with distal occlusion devices, at least 5 % of patients will not tolerate proximal occlusion.
For the sake of completeness we will briefly discuss the Enroute® Transcarotid Neuroprotection System (Silk Road Medical). This device consists of a sheath that is placed into the common carotid artery from a surgical cut-down. A vessel loop occludes the vessel proximal to the sheath. Flow is then reversed by creating a circuit between the carotid sheath and the common femoral vein. There is a filter in the circuit that traps debris. The advantages are similar to use of a proximal occlusion device. One disadvantage of this device is that it requires a surgical incision in the neck, it is not a truly percutaneous device.
Embolic Protection in Clinical Practice
The concept of trapping debris and preventing distal embolisation is appealing to endovascular specialists because it has been shown to improve patient outcomes in several vascular territories, and it intuitively makes sense that it would be of benefit in arterial vascular interventions. As previously discussed, the benefits of EPD use in coronary and carotid interventions is well accepted due to the significant clinical consequences of distal embolisation in these procedures. Due to their size and the lack of significant collateral circulation in most other vascular territories, the flow reversal devices do not have a role outside the cerebrovascular domain.
Vertebral Artery
Vertebrobasilar circulation strokes compromise approximately 20–25 % of all ischaemic strokes.6 Strokes affecting the posterior circulation carry a higher risk of recurrent stroke and death as compared with anterior circulation strokes, especially during the first seven days following a stroke or transient ischaemic attack (TIA).7 The five-year risk of subsequent stroke, after a vertebrobasilar stroke or TIA, has been reported to be 22 %.8
Surgical treatment of vertebral artery origin stenosis can be technically challenging due to access to the vessel origin. Operative mortality rates of 0.5–3.0 % have been reported and there is at least 5 % risk of post-operative occlusion.9,10 Complications include transient phrenic nerve paralysis and a mild Horner’s syndrome.
Several studies have shown the feasibility of vertebral artery stenting for treatment of stenosis at the origin and proximal, V1, segment of the vertebral artery.11–13 The use of EPDs during vertebral artery stenting has also been studied in non-randomised studies.14,15 The use of flow reversal during vertebral artery stenting has also been described.16 There are two randomised trials comparing endovascular treatment of ostial vertebral artery stenosis with medical management. In the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS)17 the outcomes were the same in both treatment arms; however, there were only eight patients in each treatment arm and therefore it is difficult to make any definitive conclusions. The Vertebral Artery Stenting Trial (VAST)18 is a randomised phase II trial that compared best medical therapy to best medical therapy and stent placement. In this study, there was a 5 % risk of major periprocedural vascular complication. The paper did not specify which of the complications were intra-procedural and did not state that EPDs were used. Other studies have reported intra-procedural stroke rates of 1 % or less without the use of EPDs when treating ostial vertebral artery lesions.19,20
The primary weakness of vertebral artery stenting is the high rate of in-stent restenosis ranging from 10 to 43 %.21–23 Lesion length does appear to play a significant role in restenosis rates in vertebral artery origin stenting.23 When restenosis rates were correlated with lesion length, restenosis occurred at a much lower rate in lesions <5 mm (21 %) versus lesions >10 mm in length (50 %). The study does not specify which stents were used, but from the discussion it may be assumed that these were bare metal stents. In a more recent metaanalysis,24 the restenosis rates for drug-eluting stents was found to be significantly lower than for bare metal stents (11 versus 30 %) in the setting of vertebral origin stenosis. Distal protection devices or flow reversal devices may provide a benefit during stenting of ostial vertebral artery lesion when plaque morphology suggests an increased risk for artery-to-artery embolism.19
Lower Extremity
The incidence of embolisation during lower extremity interventions ranges from 2 to 100 %.25–28 This wide variation in embolisation rates can be explained by differences in lesion type, lesion length, treatment modality and diagnostic criteria. When continuous Doppler ultrasound was used for monitoring interventions, distal embolisation was demonstrated during wire recanalisation, angioplasty, stent dilation and atherectomy.25 In studies evaluating the presence of macroscopic debris in the filter baskets, distal embolisation has been documented in 55–100 % of cases.28–34 Distal embolisation is most commonly encountered with the use of atherectomy devices.26 This same study documented higher rates of distal embolisation when recanalising vascular occlusions, treating in-stent restenosis and in TransAtlantic Inter-Society Consensus (TASC) C and D lesions. Patients with subacute occlusion, those <6 months in duration, may also be at higher risk for distal embolisation due to the presence of thrombus.29
The clinical significance of distal embolisation during lower extremity revascularisation is unknown. Although distal embolisation is known to require invasive treatment and even result in limb loss, many believe that clinically significant distal embolisation happens infrequently, and in most cases, it is insignificant.25 Based on the available literature, the use of EPDs should be considered in patients with TASC C and TASC D lesions undergoing angioplasty, with or without stenting, and in patients undergoing atherectomy.
Renal Artery
Renal artery stenosis (RAS) secondary to atherosclerotic vascular disease is becoming increasingly common as the population ages.35 It is a progressive condition that can lead to refractory hypertension and renal insufficiency.36 Current practice guidelines suggest benefit from percutaneous revascularisation for significant RAS in the setting of severe or persistent hypertension, ischaemic nephropathy with chronic kidney disease and cardiac disturbance syndromes (i.e. flash pulmonary oedema and acute coronary syndromes).37,38
Despite a technically successful RAS procedure, 10–20 % of patients will have continued deterioration of renal function.39,40 The aetiology behind this continued deterioration of renal function is thought to be secondary to inflammation, and microinfarction secondary to the embolisation of atheromatous debris. A review of distal protection devices for RAS ’suggested’ the use of EPDs when performing renal artery angioplasty.41
EPDs can be technically challenging to use when treating RAS due to the configuration of the renal artery. It often originates from the aorta at a right angle. RAS lesions are usually ostial and therefore the catheter system is less stable. Distal balloon and filter EPDs are designed for use in longer, narrower vessels without bifurcations. There can be poor wall apposition due to tilting of the EPD in the tortuous renal vessels and incomplete protection of the entire renal vascular bed due to early bifurcation of the renal artery. Of the available devices, the FiberNet® embolic protection system (EPS) has the smallest landing zone (1.5 cm). Its use during RAS has been described.42 When technically feasible, the use of an EPD may be beneficial. Another option for reducing cholesterol embolism is the ’No-touch’ technique.43
Mesenteric Artery
Mesenteric ischaemia can be acute or chronic. Acute mesenteric ischaemia (AMI) is most often secondary to a remote embolism.44 Surgery is traditionally the treatment of choice for AMI due to the need for visual examination of the bowel to determine viability and subsequent surgical resection of infarcted segments. Chronic mesenteric ischaemia (CMI) typically results from long-standing atherosclerotic vascular disease involving two or more mesenteric vessels.45 Despite the lack of any prospective trials comparing endovascular therapy with surgical bypass, mesenteric artery stenting (MAS) has become the most frequently used method of revascularisation to treat CMI.46 Though requiring more re-interventions for restenosis and symptom recurrence,47 MAS may offer a significant reduction in patient mortality (15 % with open bypass versus 4 % with MAS).48
In a recent study using EPDs in the setting of MAS, embolic material was identified in the EPD in 66 % of patients.49 Risk factors associated with distal embolisation include vessel occlusion, severe calcification and lesion length >30 mm.50 When these lesions characteristics are present, EPD use should be considered.
Technical Notes
As a result of EPDs being designed for coronary and cerebrovascular use, they are built on a 0.014” platform. These devices often lack guidewire support when intervening on ostial lesions of the renal and mesenteric vessels and when using 0.035” balloon and stent systems. In these cases a second 0.014” or 0.018” ’buddy wire’ can be advanced alongside the EPD. The buddy wire stabilises the catheter system while a balloon or stent is advanced into position. When using a 0.014” stenting platform and a buddy wire, it is important to keep track of the wires, advance the stent over the filterwire and remove the buddy wire prior to deployment of the stent. Failure to remove the buddy wire can result in inadvertently ’jailing’ the buddy wire.51 Worse yet, deployment of the stent on the buddy wire can result in ’jailing’ the filterwire.52 In the case of a 0.035” stent system, the stent or balloon can be advanced over both wires to provide support.
In some patients the renal or mesenteric arteries can have a sharp downward angle as they branch from the aorta. This occurs most commonly with the superior mesenteric artery. In these cases a radial approach may facilitate the procedure by providing a more inline approach to the vessel thus affording greater stability to the catheter system when attempting to engage and cross lesions, especially ostial lesions. There are 6-Fr and 7-Fr expandable sheaths that are designed for use in the radial artery as well as large diameter balloon-expandable stents mounted on balloons with long shafts that will reach the abdominal vessels from a radial approach. In cases in which the stent deployment system does not reach the target, a brachial approach can be used, but with a slightly increased risk of vascular complication.
When performing vertebral artery stenting, a buddy wire can be advanced distally in the subclavian artery to provide additional support for the catheter system. In cases where the angle of the vertebral artery is challenging to access from the femoral approach, the retrograde approach, either brachial or radial, should be considered. This approach can be particularly advantageous when treating a right vertebral artery stenosis with an elongated, type 3, aortic arch.
Conclusion
EPDs have the potential to decrease distal embolisation during vascular procedures. Unfortunately there are few prospective studies evaluating the clinical significance of distal embolisation outside of the carotid and coronary territories. Until further prospective randomised trials are published, the limited data would seem to indicate that in lower extremity interventions and mesentric interventions, there are lesion characteristics that make some lesions at greater risk for distal embolisation. In those cases, use of EPDs may be warranted. There is even less data available for renal artery interventions. If it is technically feasible, EPDs may be useful. In the vertebral artery circulation, there is also little data to support the use of EPDs; however, as in the anterior cerebral circulation, the consequences of distal embolisation can be catastrophic and therefore the use of EPDs when treating vertebral artery lesions should be considered when feasible.