Dr Edelman is the Thomas D and Virginia W Cabot Professor of Health Sciences and Technology at MIT, Professor of Medicine at Harvard Medical School, and Senior Attending Physician in the coronary care unit at the Brigham and Women’s Hospital in Boston. His team has pioneered basic findings in vascular biology and the development and assessment of biotechnology. Dr Edelman directs the Harvard-MIT Biomedical Engineering Center, dedicated to applying the rigors of the physical sciences to elucidate fundamental biological processes and mechanisms of disease. Dr Edelman is a founding member of the A-CURE Working Group.
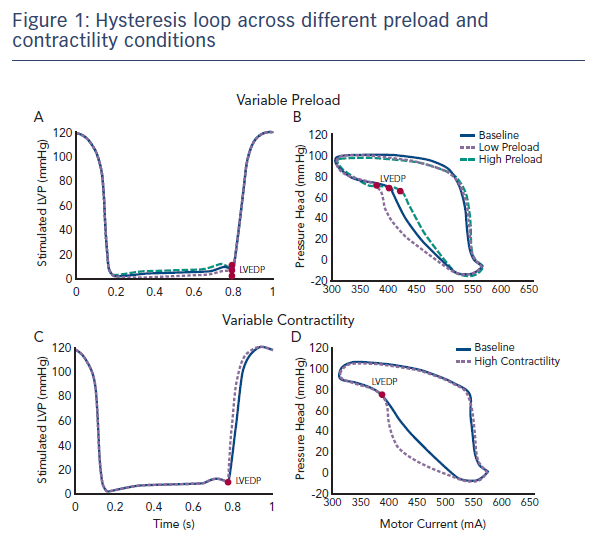
Dr Edelman commenced his presentation by reminding the audience that the Impella can be utilised not only as a therapeutic tool, but also as a diagnostic tool. Since it resides in the ventricle, it is uniquely positioned to provide insights into the function of the heart. It works in concert with the heart, is relatively non-disruptive, and its design allows the extraction of a substantial amount of information. The pump maintains a constant rotor speed by changing the motor current in response to the variable load caused by a pulsatile flow envirwonment. The motor current undergoes subtle variations in every beat. Dr Edelman’s team therefore proposed the hypothesis that the relationship between a pump parameter (motor current) and a physiological parameter (pressure head) could be used to obtain diagnostic information on the function of the left ventricle (LV). The plotted relationship between the pressure head and the motor current forms a hysteresis loop which is asymmetric, non-linear and changes with each cycle, making extraction of information challenging. However, every pump exhibits the same hysteresis loop phases, making it possible to extract information about heart function such as the LV end diastolic pressure (LVEDP), peak pressure, slope of relaxation and slope of contraction.
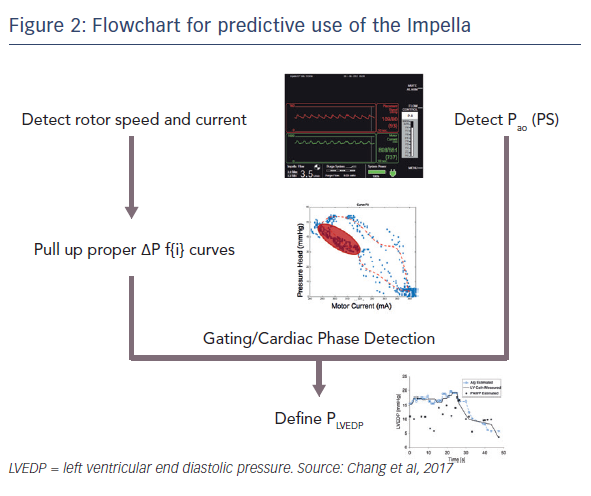
In a recent study that aimed to validate this hypothesis, an Impella CP with both ventricular and aortic pressure sensors was implanted into a mock circulation loop.1 The contractility was kept constant and the LVEDP was varied (see Figure 1). Similarly, it was possible to maintain a constant preload and vary the contractility. The motor current and aortic pressure were extracted from the console and plotted to illustrate the hysteresis relationship (see Figure 1). These inputs were then used to calculate LVEDP and contractility measurements. Using these techniques, multiple indices of LV function may be measured. Dr Edelman presented a flow chart describing the method for the use of device extracted metrics to predict physiological function, such as LVEDP during (see Figure 2).1 It is hoped that this approach to estimating metrics of heart function will be placed into next generation of Impella devices.
Swan-Ganz catheters are used in patients regularly to estimation of LVEDP with real-time wedge tracing during end-expiratory hold. This new method of estimating heart function using device derived metrics would decrease the number of catheters in patients on Impella support by having one catheter residing in the LV that provides both circulatory support and heart function information.
In summary, understanding the dynamic changes of disease progression and its effect on cardiac state allows for standardised care of the patient, as well as improved outcomes using quantitative optimisation. From the clinician point of view, it also allows determination of the optimal Impella therapy in conjunction with other forms of therapy.