This presentation discussed the comparison of imaging tools from ACIST and Boston Scientific, together with optical coherence tomography (OCT). Dr Chieffo has found IVUS particularly useful in the assessment of multivessel disease and complex lesions. The importance of IVUS guidance in PCI is well established. A meta-analysis of 24,849 patients from three randomised controlled trials (RCTs) and 12 observational studies between 2005 and 2013 that compared IVUS- and angiography-guided PCI showed that IVUS was associated with a lower rate of major adverse cardiac events (odds ratio [OR] 0.79, 95 % confidence interval [CI] [0.69–0.91]; p=0.001). IVUS-guided PCI was also associated with significantly lower rates of all-cause mortality (OR 0.64; 95 % CI [0.51–0.81]; p<0.001), MI (OR 0.57; 95 % CI [0.42–0.78]; p<0.001), TVR (OR 0.81; 95 % CI [0.68–0.95]; p=0.01) and stent thrombosis (OR 0.59; 95 % CI [0.42–0.82]; p=0.002). The authors concluded that IVUS guidance is associated with a significant reduction in adverse events following PCI when compared with angiography guidance alone.4
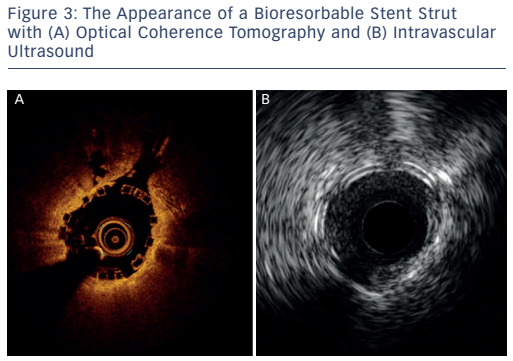
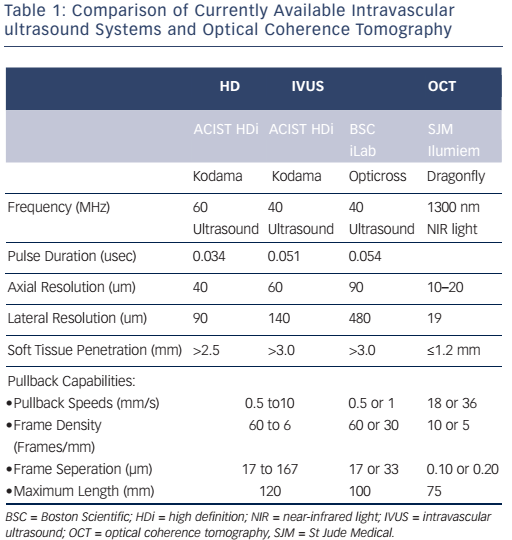
The imaging tools available in the Milan laboratory are ACIST high-definition IVUS system with a 40 and 60 MHz operation mode, Boston Scientific 40 MHz iLab™ and OCT. Table 1 shows the key features of these systems. Dr Chieffo presented three clinical cases to illustrate the differences between the systems. In the first case, highdefinition IVUS was compared to OCT in a typical patient with diffuse left anterior descending artery disease. The procedure involved the use of bioresorbable vascular scaffolds (BVS) and drug-coated balloon catheters. With these new technologies, accurate imaging tools are essential to guide procedures and to decide which stent is the most appropriate for the patient, if they are suitable for BVS and to assess the final result. Figure 3 shows the difference in images obtained by IVUS and OCT. With the40 MHz system, the strut was not easy to detect. The OCT imaging was superior in terms of determining overlapping, gaps in the BVS, branch coverage, malapposition, dissection behind the struts and asymmetric expansion with calcification. However, the use of OCT requires additional contrast and is considerably more expensive than IVUS, therefore cost-effectiveness is a factor when selecting imaging modalities.
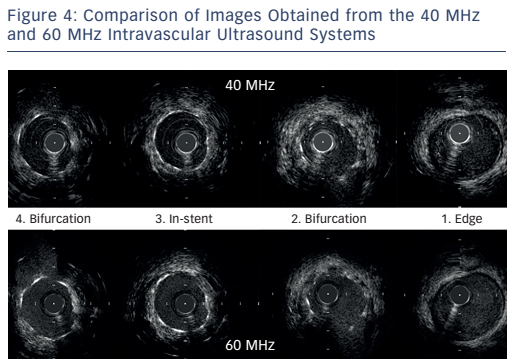
The next case was a comparison of the high-definition IVUS 60 MHz versus the Boston Scientific 40 MHz IVUS during BVS implantation. Images at different points of the affected vessel were compared. The images obtained by the 60 MHz system are not equal to the quality of OCT but are superior to the 40 MHz system and can help in decision-making. Importantly, the 60 MHz system allows imaging of the BVS without the need for contrast clearing. Another case was presented of a complex bifurcation lesion in which a drug-eluting stent was implanted. The images from the 60 MHz system are clearly superior in quality compared with the 40 MHz system (see Figure 4). In conclusion, in routine imaging, more data are needed to guide complex interventions. The 60 MHz high-definition IVUS system may become a cost-effective means of providing superior images.