Dr Tschöpe is a Professor of Medicine and Cardiology and the Vice Director of the Department of Cardiology, Charité, CVK, Berlin, guiding the cardiomyopathy programme. His main research interests are the potential of cell therapies to cure heart failure and the role of the immune system in heart failure.
Acute fulminant myocarditis and giant cell myocarditis have a poor prognosis.1 At present, short-term mechanical circulatory support (MCS) for myocarditis patients with refractory cardiogenic shock (CS) has predominantly used extracorporeal membrane oxygenation (ECMO),2 despite the use of Impella CP® for all other CS situations. Several case reports of successful short-term use of the Impella in fulminant myocarditis and giant cell myocarditis have been published.3–7
These reports have suggested that peripheral unloading with the Impella may not be merely providing circulatory support, but it also may be conferring additional anti-inflammatory effects that modify the disease state, thus allowing a bridge to recovery in patients with fulminant myocarditis. In order to explore this hypothesis further, Dr Tschöpe presented the known pathophysiological processes underlying myocarditis, including pro-inflammatory and fibrotic processes that lead to cardiac remodelling and failure during disease progression. In an overloaded myocardium such as is present during fulminant myocarditis, mechanical stress activates integrins (mechanoreceptors) in the heart, which are known to mediate pro-inflammatory and fibrotic processes. Furthermore, integrins are known to have direct detrimental effects on the contractile apparatus. These combined effects exacerbate myocarditis, and contribute to the observed poor patient outcomes. Therefore, the hypothesis is logically raised whether haemodynamically unloading the heart by means of MCS (thereby decreasing mechanical stress) is a sufficient means to overcoming these pathophysiological mechanisms.
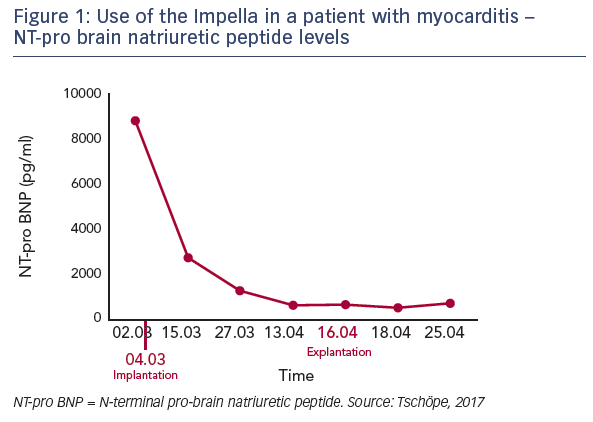
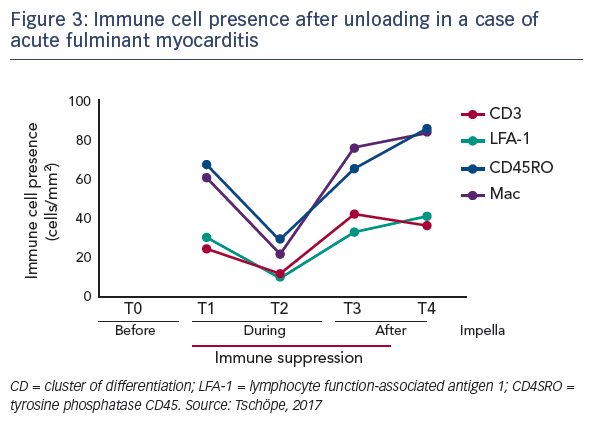
The hypothesis that mechanical unloading may improve reverse remodelling in fulminant myocarditis was tested in a 62-yearold patient recently admitted to the practice of Dr Tschöpe with severe myocarditis and pre-CS despite immunosuppressive therapy. An axillary Impella 5.0 was implanted, which remained in place for 40 days. After 2 days in bed, the patient was mobile. Steroid therapy and unloading gave a significant improvement in ejection fraction from 5 days after implantation. In addition, the patient’s NT-pro brain natriuretic peptide levels reduced over time (see Figure 1), and increases were seen in EF and global longitudinal strain during short-time loading (see Figure 2). After 4 weeks, an echocardiogram showed the first signs of recovery.
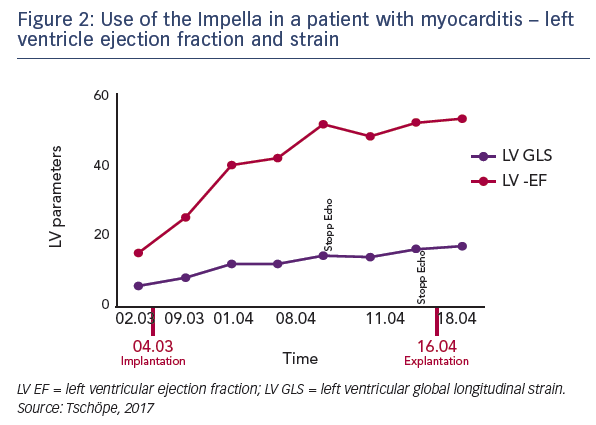
Serial left ventricular biopsies taken at various time points during treatment to assess biomarkers of inflammation. These data demonstrate that the inflammatory response was significantly reduced during the time of Impella support. During this time steroids were also applied to decrease the inflammatory response. However, importantly, when the Impella was removed, the inflammatory response significantly increased, despite continued steroid use (see Figure 3). This suggests that Impella support may provide clinically important additional antiinflammatory benefits beyond that observed with steroids alone. This pattern was seen for the levels of adhesion molecules ICAM-1 and VCAM-1, and also integrin receptors. Mass spectroscopic analysis of the biopsy samples revealed significant changes in protein composition, notably in the matrix proteins collagen and vimentin, which are important for integrin function. There was also a rapid improvement in titin function after unloading, which is essential for maintaining the elasticity of cardiomyocytes. Finally, energy consumption was assessed by measurement of glucose uptake and mitochondrial malate dehydrogenase. Again, a significant improvement was seen during the period of mechanical unloading.8
In conclusion, experience to date supports the hypothesis that prolonged unloading with an Impella device offers circulatory support with additional disease-modifying effects that are important for bridging fulminant myocarditis patients to recovery.