Percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) are established strategies for revascularisation in patients with coronary artery disease. While CABG was the standard of care for patients with multivessel disease, the introduction of baremetal stents (BMSs) and, later, drug-eluting stents (DESs) has led to an increased use of PCI in these more challenging cases. The latest generation of polymeric metallic DESs has shown improved efficacy and safety compared with BMSs and first-generation DESs, facilitating the treatment of more complex lesions.1 However, certain lesions and patient subsets remain difficult to treat. Patients with diabetes represent a particular challenge as they have rapidly progressing and more diffuse coronary artery disease, with lesions of longer length. They therefore continue to have worse outcomes following PCI compared with non-diabetic patients.2 In patients with diabetes and multivessel disease, CABG remains the preferred strategy but it is associated with a higher risk of peri-procedural stroke.3,4
Diabetes has reached epidemic proportions worldwide and its prevalence is rising. In 2015, 415 million adults had diabetes; nearly half (46.5%) of these remain undiagnosed.5 By 2040 this number is projected to rise to 642 million,5 representing a major health and economic burden. There is an urgent need to enhance procedural success and long-term clinical outcomes in the coronary revascularisation of patients with diabetes. In particular, there is a need for improved stent technology tailored to the needs of the diabetic population. This article describes the Cre8™ DES, which has a novel design that may provide particular efficacy and safety advantages in patients with diabetes.
Introduction to the Cre8™
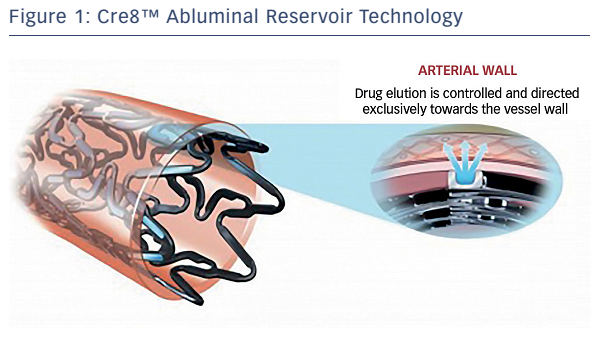
The Cre8™ has unique features that may specifically improve clinical outcomes in patients with diabetes. These include abluminal reservoir technology, which is a proprietary polymer-free drugrelease system consisting of reservoirs on the stent’s outer surface that control and direct drug release exclusively towards the vessel wall (see Figure 1). The reservoir’s design directly impacts on the drug dosage and release kinetics, allowing peak drug tissue concentration during the first days post-implantation, 50 % drug elution in approximately 18 days, 65–70 % elution within 30 days and complete drug elution within 90 days.6
The polymer-free system overcomes a major challenge of DES design. Polymer coatings have been routinely used to control drug release and optimise DES efficacy, but have also been implicated in late inflammatory reactions and delayed arterial healing after stenting.7 The Intracoronary Stenting and Angiographic Restenosis Investigators – Test Efficacy of Rapamycin-eluting Stents with Different Polymer Coating Strategies (ISAR-TEST-3) study found that failure to retard drug release results in suboptimal antirestenotic activity.8
A second design characteristic unique to the Cre8™ is the Amphilimus™ formulation of the active antirestenotic drug. In this proprietary technology, sirolimus and a fatty acid are eluted together, allowing sustained drug elution, modulated drug bioavailability, a more homogeneous drug distribution and enhanced drug stability. Also important is the bioinducer surface, an integral ultra-thin (<0.3 μm) pure carbon coating that is covalently bonded to the thin CoCr platform (total thickness 70–80 μm). This is hypothesised to passivate stent–platelet interactions (since the bulk of the CoCr platform is sealed by the coating) and reduce the risk of thrombotic events. Other features of the Cre8™ include homogeneous stent design in lengths from 8 to 46 mm, excellent longitudinal stability on expansion, and two platinum markers at the stent ends.
Challenges of DES Use in Patients with Diabetes
The use of second-generation DESs in patients with diabetes has been associated with suboptimal outcomes. A pooled patient analysis of data from four randomised trials – the Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Patients with de novo Native Coronary Artery Lesions (SPIRIT) II, SPIRIT III, SPIRIT IV trials, and the Second-Generation Everolimus-Eluting and Paclitaxel-Eluting Stents in Real-Life Practice (COMPARE) trial – found that, in the non-diabetic population (n=3,911), the use of an everolimus-eluting stent (EES) significantly reduced the endpoints of cardiac death or myocardial infarction (MI) at 1 year (4.3 versus 9.5 %; HR 0.44; 95 % CI [0.35–0.55]; p<0.001) compared with a paclitaxeleluting stent (PES). However, in the diabetic population (n=1,869), there was no difference between the two DESs (8 versus 7.8 %; HR 1.01; 95 % CI [0.72–1.42]; p=0.95). In addition, the incidences of death, MI and target-lesion revascularisation were all higher in diabetic than in non-diabetic patients.9 Furthermore, in the Randomized Comparison of Everolimus-Eluting Stent versus Sirolimus-Eluting Stent Implantation for De Novo Coronary Artery Disease in Patients with Diabetes Mellitus (ESSENCE-DIABETES), 300 patients with diabetes were randomised to receive either EES or sirolimus-eluting stents (a first-generation DES) for the treatment of coronary lesions. No difference in outcomes was seen between the two groups.10
Basic research has shed additional light on the reasons for the lack of benefit second-generation DESs have shown in patients with diabetes. The mammalian target of rapamycin (mTOR) inhibitors (-limus drugs) have been found to be less active in patients with diabetes compared with the general population. One reason is the direct resistance of vascular smooth muscle cells to mTOR inhibition.11,12 Dose-response curves show that a tenfold higher concentration of mTOR inhibitor is required in the diabetic cell to achieve similar inhibition to the non-diabetic cell.12 Another reason for the relative ineffectiveness of -limus drugs in diabetic patients is the effect of other hormones. BMI strongly correlates with diabetes riks: 90 % of patients with type 2 diabetes are overweight.13 Human obesity is associated with elevated levels of the hormone leptin, which has been found to promote vascular remodelling and neointimal growth in animal studies.14 Increased leptin levels cause a ninefold increase in the dose of sirolimus required for effective inhibition of neointimal formation.15 Moreover, leptin levels have been associated with in-stent restenosis.16,17
These data highlight the potential unmet need with regard to currentgeneration DES technology in patients with diabetes. Increasing total drug delivery to the vessel wall is one approach. This may be achieved by incorporation of an increased drug–polymer load, although this is likely to negatively impact overall vessel wall healing. Another possibility would be to use the same coating thickness with an increased drug:polymer ratio, but this would negatively affect the drug-release kinetics and make the polymer brittle. Placing the drug directly onto the bare metal would also be problematic in terms of control of drug release. An optimum approach might be to use standard drug–polymer loads combined with a formulation targeted to enhance uptake of drug at the cellular level.
The Amphilimus™ formulation of Cre8™ is designed to take advantage of the key role of fatty acids in cellular metabolism in patients with diabetes. In the non-diabetic cell, fatty acid metabolism is responsible for 70 % of the generation of adenosine triphosphate, the remaining 30 % being produced by glucose oxidation. However, in the diabetic cell, membrane protein overexpression leads to higher binding and translocation of fatty acids, and as a result 100 % of adenosine triphosphate production results from fatty acid oxidation.18 In diabetic mouse models, a doubling of cardiac fatty acid uptake has been demonstrated.19 This technique of using fatty acids has been shown to enhance the transdermal delivery of other drugs.20
Clinical Evidence for Use of the Cre8™
The first clinical study of the Cre8™ was the International Randomized Comparison between DES Limus Carbostent and Taxus Drug-eluting Stents in the Treatment of de novo Coronary Lesions (NEXT) clinical study, which randomly allocated patients (n=323) to the Cre8™ or a PES (TAXUS® Liberté®, Boston Scientific).21 The primary endpoint was 6-month angiographic in-stent late lumen loss (LLL). At 6 months, the Cre8™ group had a 60 % reduction in in-stent LLL compared with the PES group (0.14 ± 0.36 mm versus 0.34 ± 0.40 mm; p<0.0001). This finding was even more pronounced in the subgroup of patients with diabetes (n=82), where a 72 % reduction in in-stent LLL was seen (0.12 ± 0.29 mm versus 0.43 ± 0.41 mm; p<0.0001). In terms of 3-year major adverse cardiac events (MACE), a 37 % reduction in target lesion revascularisation (TLR) was seen in the Cre8™ versus the PES group (6.9 versus 10.9 %; p=0.2464) in the overall population and a 68 % reduction in the subgroup with diabetes (6.8 versus 21.2 %; p=0.888).21,22
These findings led to the initiation of a study enrolling patients more representative of those seen in routine clinical practice. The Prove Abluminal Reservoir Technology clinICal benefIt in all-comer PATiEnts (pARTicip8) study recruited ‘real world’ patients with ischaemic myocardial symptoms due to de novo lesions in native coronary arteries from across 30 European sites. The objective of this study was to evaluate the safety and efficacy of Cre8™ in a group of patients comparable to the everyday clinical practice population, with a specific focus on subgroups with diabetes. The primary endpoint was a composite of cardiac death, target vessel MI and clinically-indicated TLR 6 months after the index procedure. Of those enrolled (n=1,186), 308 (25.8 %) had diabetes. At 1 year, the incidence of clinically-indicated TLR was 1 % in the overall population and 1.4 % in the diabetic subgroup. The LLL in the diabetic subgroup was 0.16 ± 0.13. Definite late stent thrombosis was detected in only 0.08 % of the patient population.22 By contrast, most other DESs have shown a significantly higher LLL in people with diabetes compared to those without.10,21,23,24
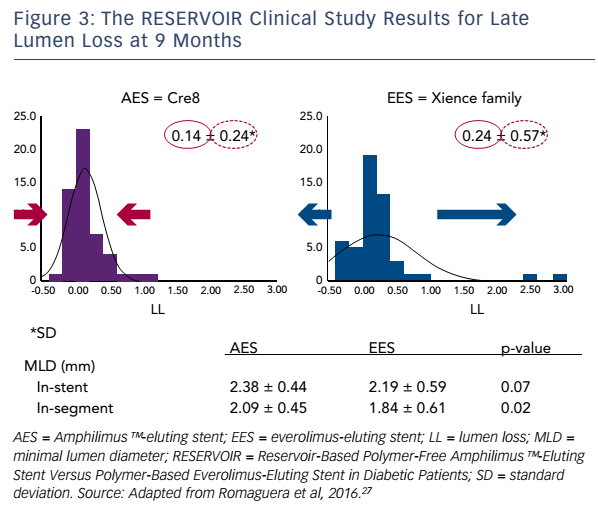
The next step in the Cre8™ clinical development programme was the retrospective MultIceNtric and RetrospectiVe REgiStry in 'real world' paTients with polymer-free drug elutInG stent Cre8™ (INVESTIG8) study. The primary endpoint was the incidence of a clinical composite endpoint (cardiac death/target vessel MI/clinically-driven TLR) at 12 months from the index procedure.25,26 Secondary endpoints comprised the incidence of a clinical composite endpoint (all deaths/all MI/any revascularisation) from the time of the index procedure to 12 months; and the incidence of stent thrombosis from the index procedure to 12 months, classified according to the Bleeding Academic Research Consortium definition (see http://bit.ly/2eemnyq). Twelve-month followup data were available for 589 out of the 647 patients enrolled (91 %). Of these, 47.4 % had diabetes. Chronic total occlusions were present in 3.4 %, ostial lesions in 15.5 %, bifurcation in 17.8 % and de novo lesions in 93.8 % of participants. The composite endpoint occurred in 4.2 % of patients, and clinically-indicated target vessel revascularisation in 1.5 %. In a sub-analysis of non-diabetic versus diabetic patients, only the composite endpoint was higher in patients with diabetes (5.0 versus 3.5 %; p=0.4863), and this was due to the expected higher incidence of cardiac death and MI. However, the incidence of clinically-indicated TLR was not higher in the subgroup with diabetes (1.6 versus 1.4 %; p=1.000; see Figure 2).
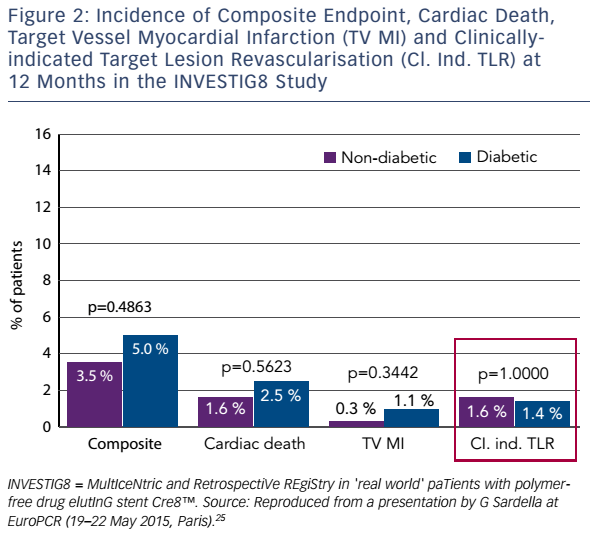
The Randomized Comparison of Reservoir-Based Polymer-Free Amphilimus™-Eluting Stent Versus Polymer-Based Everolimus-Eluting Stent in Diabetic Patients (RESERVOIR) clinical trial enrolled only patients with diabetes receiving glucose-lowering agents.27 The 112 participants were randomised either to Cre8™ or an EES (XIENCE®, Abbott Vascular). The primary endpoint – mean neointimal hyperplasia volume obstruction – was 11.97 ± 5.94 % in the Cre8™ group and 16.11 ± 18.18 % in the EES group (noninferiority p=0.0003; superiority p=0.22). Prespecified subgroup analysis showed consistent treatment effect in favour of the Cre8™ arm across all subgroups. In terms of secondary endpoints, LLL in the Cre8™ arm was 0.14 ± 0.24 mm compared with 0.24 ± 0.57 mm in the EES arm. The difference between the standard deviations is striking, and demonstrates the consistent performance of the Cre8™ (see Figure 3). The minimal lumen diameter was also higher in the Cre8™, both in-stent (2.38 ± 0.44 mm versus 2.19 ± 0.59 mm; p=0.07) and in segment (2.09 ± 0.59 mm versus 1.84 ± 0.61 mm; p=0.02).
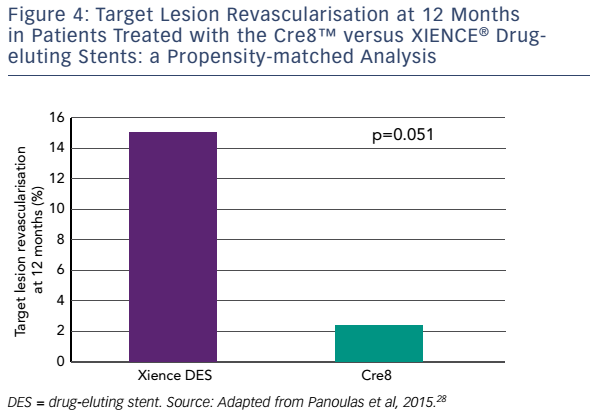
Finally, a matched analysis has been performed in which 187 consecutive patients treated with Cre8™ between January 2011 and August 2013 in four Italian centres were propensity matched with 150 patients treated with new-generation EESs during the same period. The incidence of 1-year estimated MACE was numerically lower in Cre8™ compared to the EESs, although the difference was not statistically significant (7.4 versus 10.2 %, respectively; p=0.261). The same was true of allcause mortality (1.3 % Cre8™ versus 1.4 % EES; p=0.823), target vessel revascularisation (5.2 % Cre8™ versus 8.8 % EES; p=0.169) and TLR (3 % Cre8™ versus 7.4 % EES; p=0.108). Subgroup analysis showed that these differences were more pronounced in patients with diabetes, with a 50 % decrease in the incidence of MACE, although this was not statistically significant (10 % Cre8™ versus 20 % EES; p=0.204). Of particular note, in patients with diabetes (Cre8™, n = 42; EES, n = 41), 1-year TLR was 2.5 % in the Cre8™ group versus 14.6 % in the EES group, which represented an 83 % reduction (p=0.05; see Figure 4).28
While a growing body of clinical and real-world data has provided support for the efficacy of the Cre8™, studies have been underpowered to demonstrate statistical superiority over other DESs. To do so, a large clinical study is planned: the Clinical benefit in ‘all comers’ patients with DIABetes to prove Cre8™ DES superior efficacy (DIAB8).29 In this study, all-comer patients with diabetes undergoing PCI will be randomised in a 1:1 ratio to the Cre8™ or to an everolimus DES. The primary endpoint will be 12-month target lesion failure. Around 2,200 patients from 50 international sites will be recruited and clinical follow-up will be at 1 and 3 years.
Safety
In addition to these efficacy data, the Cre8™ has demonstrated excellent safety. In the INVESTIG8 study, the incidence of definite stent thrombosis at 12 months was low in both non-diabetic and diabetic patients (0.3 % in each group). The ranDomizEd coMparisOn betweeN novel Cre8™ DES and BMS to assess neoinTimal coveRAge by OCT Evaluation (Demonstr8) study was designed to assess whether strut coverage 3 months after implantation of the Cre8™, once it becomes a BMS after complete drug-elution, was equivalent to a standard BMS.30 Patients (n=38) with ischaemic myocardial symptoms related to de novo lesions in native coronary arteries were randomised 1:1 to receive a Cre8™ or a BMS (VISION®, Abbott Vascular). The primary endpoint of ratio of uncovered to total stent struts per cross section (RUTTS) score <30% occurred in 99.8% of Cre8™ struts and in 99.6% of the BMS struts (p for noninferiority <0.001).
The first results of a single centre registry study undertaken in Utrecht (U-Cre8) have recently been presented.31 This study involved 332 coronary lesions in 201 patients with diabetes and accrued data from 2012 to 2014. A retrospective analysis propensity-matched 99 patients receiving the Cre8™ with 102 patients receiving a zotarolimus-eluting stent (ZES). The mean duration of dual antiplatelet therapy following PCI was 4.8 ± 3.4 months in the Cre8™ group versus 5.9 ± 4.1 in the ZES group. Target-lesion failure occurred in six (6.1 %) patients in the Cre8™ group compared to 11 (10.7 %) in the ZES group. These failures were attributable to TLR in three Cre8™ patients (3.0 %) and seven ZES patients (6.9 %). End MACE-free survival was reported in 93 (93.9 %) Cre8™ patients compared to 87 (85.2 %) of the ZES group at study end. It must be stressed, however, that these are preliminary data that still require independent adjudication.
The Prospective, Single Center, Open Label, Randomized Controlled, Two Arm Study Evaluating Safety and Efficacy of the Permanent Polymer Zotarolimus Eluting Stent Resolute Integrity Compared to the Polymer Free Amphilimus™-Eluting Stent Cre8™ (ReCre8) is currently recruiting participants. In this all-comer study, patients (n=1,530) with ischaemic myocardial symptoms are randomised to the Cre8™ or the Resolute DES (Medtronic).32 The study is also investigating the use of dual antiplatelet therapy for 1 month in elective PCI. Clinical follow-up is at 1, 2 and 3 years, and the primary endpoint is MACE at 12 months.
Excellent safety and efficacy data have been reported in support of the Cre8™, and results of the ReCre8 study are awaited.
Conclusion
Poorer outcomes following PCI reflect an unmet need in stent technology for patients with diabetes. The lower efficacy of DESs in these patients is largely due to reduced responsiveness of cells to -limus drugs. The Cre8™ utilises a proprietary polymer-free drugrelease system consisting of reservoirs on the stent’s outer surface, maximising drug delivery without increasing the dose. The Amphilimus™ formulation enhances cellular drug uptake, particularly in diabetic cells. Finally, clinical trial and real-world data have demonstrated the superior performance of the Cre8™ compared with other DESs in diabetic subgroups, as well as remarkable consistency in terms of LLL. These findings suggest that the Cre8™ is a useful option in PCI in this challenging patient population. Data from on-going studies are eagerly anticipated.